Fax: 770-339-9804
Lawrenceville, Georgia 30046

Originally published on PRS Global Open website in April 2021
Abstract
Summary
Patients with central breast cancers historically underwent mastectomy, as the aesthetic implications of removing the nipple–areola complex and central breast tissue left disfiguring defects after breast conservation. The introduction of oncoplastic techniques allowed for central lumpectomies in ptotic patients, as the excess skin and gland could be mobilized centrally to fill the defect and even immediately reconstruct a nipple and areola. These reconstructions used excess skin on the Wise pattern vertical limbs to create a nipple, or on a “neopedicle” where both the areola and nipple were reconstructed and then mobilized superiorly into position as would be performed for a conventional mastopexy or reduction. These techniques importantly allowed for the immediate reconstruction of a nipple and areola in patients who often imminently required radiotherapy and where subsequent surgery would be challenging. Here we describe another option for immediate nipple and areola reconstruction in these patients—nipple sharing and a skin graft—a well-established approach in post-mastectomy patients but never previously described for patients undergoing breast conservation.
Introduction
The nipple–areola complex (NAC) is a critical component of breast aesthetics.1 Patient reported outcomes after NAC reconstruction were associated with higher levels of satisfaction in comparison with those who choose to forego this additional step.2,3 Multiple techniques have been described to reconstruct the NAC, most commonly after mastectomy, often as the final step in the reconstructive process.4 NAC reconstruction after breast-conserving surgery has not been extensively reported on, as most patients with central breast cancers have historically undergone mastectomy. In these patients, removal of the NAC and underlying breast tissue often left defects that deformed the breast and were further exacerbated by radiotherapy.
The introduction of level-2 oncoplastic breast-conserving surgery in patients with ptosis changed our approach to central breast cancers.5 Grisotti6 described a vertical mastopexy approach where excess inferior breast skin and tissue were mobilized to replace the NAC with a circular disk of skin, avoiding deformity and reshaping the breast. Since then, Wise-pattern approaches have been described where a nipple is immediately created from one of the vertical limbs7 or the entire NAC is reconstructed on an inferior pedicle of tissue.8 Immediate reconstruction of the NAC after breast conservation is appealing because we can avoid delayed surgery in a radiated field and, as opposed to mastectomy surgery, the skin flaps and tissues are well vascularized and can support an immediate NAC reconstruction.
The nipple sharing technique has been well described after mastectomy in the delayed setting.9 This typically requires a contralateral nipple that can contribute 50% or more of its volume. This technique allows for symmetry with regard to color and texture. While bilateral nipple reconstructions are often symmetrical, this is difficult to achieve after unilateral mastectomy because a reconstructed nipple from local skin flaps tends to have a significantly different appearance (size, shape, color, and texture) than a native nipple. Matching a reconstructed nipple to the native nipple is our major technical challenge when reconstructing the NAC after breast-conservation surgery and why the nipple sharing approach is well suited for NAC reconstruction after central lumpectomy. In patients with smaller nipples, nipple sharing can still often provide an excellent result in comparison with local flap techniques.
Methods
Patients with ptosis and central breast cancers that encroach upon the nipple are evaluated for nipple reconstruction after Wise-pattern central lumpectomy (Fig. 1A). The best candidates are patients with large contralateral nipples that can tolerate a 50% decrease in size. After excision of the NAC and underlying cancer, the residual skin within the Wise-pattern is deepithelialized to facilitate involution of the residual tissue and reconstruction of the central defect. The breast is then closed using the Wise-pattern followed by contralateral mammaplasty. In 3 patients, a thoracodorsal artery perforator flap was also utilized to replace volume after resection of a multicentric cancer. The contralateral nipple is evaluated for division in half in the erect state. Tubular, overly-projecting nipples are divided in a coronal plane and closed with a purse-string absorbable 5-0 suture, whereas more broadly-based nipples with less projection are divided in a sagittal plane and primarily closed.9 The harvested graft is placed on a deepithelialized dermal bed and sutured in place with multiple interrupted 5-0 absorbable suture (Fig. 1B). The full-thickness skin graft is then sutured to the dermal bed with absorbable 4-0 suture, making a slit to accommodate the nipple and trimming excess skin to facilitate an even transition between the reconstructed nipple and areola (Fig. 1C). Petroleum gauze bolsters are then secured over the reconstructed NAC and removed 7 days later. We typically obtain excellent symmetry between the reconstructed and native nipples (Fig. 1D). (See figure 1, Supplemental Digital Content 1, which displays (A) A 77-year-old woman with multicentric left breast ductal carcinoma in situ spanning 7 cm and bloody nipple discharge. Preoperative imaging demonstrates calcifications streaming into the left nipple. She desires breast conservation and immediate nipple reconstruction. (B) She is seen here 16 months after the completion of left breast radiotherapy. She is reconstructed using a Wise-pattern volume displacement technique and addition volume supplementation with a thoracodorsal artery perforator flap. Her left nipple and areola is immediately reconstructed with half of the contralateral nipple and excess skin from the left breast, respectively. She undergoes immediate right mastopexy as well for symmetry. https://links.lww.com/PRSGO/B630.) (See figure 2, Supplemental Digital Content 2, which displays (A) A 57-year-old woman with a right breast cancer involving the nipple and areola. She has a history of bilateral saline subpectoral breast augmentation. She desires breast conservation, immediate nipple reconstruction, and maintenance or revision of her breast implants. Her nipples are not large enough for nipple sharing, but we feel this technique will still give us the best symmetry in comparison with the use of a local flap. (B) She is shown here 6 months after surgery. She is recommended to proceed with radiotherapy but refuses. She is reconstructed with a Wise-pattern volume displacement technique, exchange of her saline implants for silicone prosthetics, and contralateral mastopexy. She undergoes reconstruction of her right nipple and areola using nipple sharing from the left breast and excess skin from the right. Despite having minimal projection of both nipples, her symmetry is very good, which would be difficult to achieve with local flap techniques. https://links.lww.com/PRSGO/B631.)
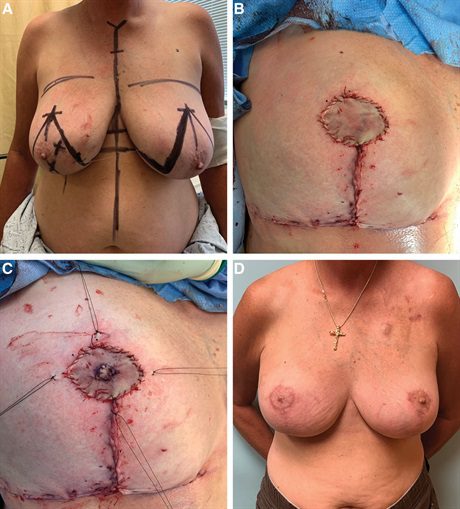 Fig. 1.:
Fig. 1.:
A 51-year-old woman with multicentric right breast cancer with bloody nipple discharge and tumor abutting the nipple–areola complex. A, Preoperative view. The patient desired breast conservation and immediate nipple reconstruction. B, Her right breast cancer was resected, and her breast was immediately reshaped using Wise-pattern volume displacement techniques. We harvested excess skin from within the Wise pattern and used this to immediately reconstruct the areola. The skin was defatted and then placed onto the deepithelialized dermal bed. C, The contralateral nipple was divided in two and primarily closed. The harvested nipple was placed in the center of the dermal bed and sutured down and to the surrounding skin graft. A petroleum gauze bolster was used to secure the reconstructed nipple and areola, and was removed after 7 days. D, The patient is shown 12 months after the completion of right breast radiotherapy with very good symmetry between the reconstructed and native sides.
All nipple reconstructions were performed in the same operative setting as that of the oncological resection. We assessed patient satisfaction with their surgical experience using a 5-point Likert scale (1 = strongly disagree, 2 = disagree, 3 = undecided, 4 = agree, 5 = strongly agree) for the following questions:
Results
Ten consecutive patients with a mean age of 53 (range, 32–74 years; SD, 8.1 years) underwent Wise-pattern central lumpectomy with immediate NAC reconstruction using the nipple sharing and skin graft technique, with a minimum of 6 months follow-up after radiotherapy (except for 1 patient who refused radiation). All patients underwent immediate contralateral mammaplasty for symmetry. The mean size of the malignancy was 2.7 cm (range, 0.9–5.6 cm; SD, 1.9 cm). The mean partial mastectomy specimen weighed 87 g (range, 30–310 g; SD, 62 g).
Three patients had a formal diagnosis of diabetes mellitus, all of whom healed without complication. One active smoker who successfully abstained from smoking before surgery healed without incident. There were no nipple graft failures but we did have 3 areola reconstructions where up to 25% of the graft was lost, all of which healed within 7 weeks of surgery. These patients were offered tattoos but none have decided to proceed thus far. Two patients (20%) had close or positive margins and were successfully re-excised. Patients underwent adjuvant radiotherapy on average 5.2 weeks after surgery (range, 4.1–7.7 weeks; SD, 1.9 weeks).
Reconstructed nipple projections were measured at the conclusion of surgery in the supine position and again at least 6 months (mean 8.6 months; SD, 2.3 months) after radiotherapy. The average reconstructed nipple projection measured 0.77 cm at surgery (range, 0.11–1.13 cm, SD, 0.42 cm). These nipples retained on average 89.2% of their projection in long-term follow-up (range, 85.7–98.6%, SD 4.4%).
Finally, we assessed patient satisfaction using a 5-point Likert scale and found that patients were satisfied with both the appearance and symmetry of their reconstructed NAC (4.62 ± 0.42) and would choose to have the same reconstruction performed if confronted with same decision again (4.54 ± 0.39).
Discussion
The nipple sharing technique has been well described after mastectomy9 but never previously described after breast conservation. After mastectomy, this approach is the most useful for unilateral nipple reconstructions because the color, texture, size, and shape must match the contralateral nipple. This is rarely accomplished using standard local flaps to reconstruct a nipple. As almost all nipple reconstructions after breast conservation are unilateral, this approach would seem especially well-suited after central lumpectomy. Nipple sharing is the only technique that can provide the same tissue to obtain a reconstruction to match color, shape, size, and texture in a single procedure. Immediate nipple reconstruction is especially important in the setting of breast conservation because these patients will undergo radiotherapy and further reconstruction will be challenging. In addition, the nipple sharing technique produces durable results as opposed to standard local flaps, where shrinkage and color change are common.4 The overwhelming majority of our Wise-pattern oncoplastic procedures also undergo immediate contralateral mammaplasty. As such, immediate nipple sharing is a natural extension of this procedure and does not require a significant decision on the patient’s part to allow for additional surgery on the healthy breast.
Conclusions
Nipple sharing and skin grafting allow for an immediate NAC reconstruction in patients undergoing central lumpectomy who require radiotherapy and where subsequent surgery would be challenging. In addition, this approach provides the best symmetry for patients undergoing unilateral NAC reconstructions, as almost all patients undergoing breast conservation do, as the nipple is reconstructed with like tissue, providing the best match of color, texture, size, and shape with durable long-term results in comparison with local flap techniques.
References
Jean-Claude Schwartz, MD, PhD – Breast Cancer and Reconstructive Surgeon

As a national leader in oncoplastic breast surgery, Dr. Schwartz has developed and published some of the most innovative ways to save women’s breasts, allowing them to avoid mastectomy and multiple reconstructive surgeries. Dr. Schwartz’s techniques have benefitted his patients and other surgeons around the world.
There is no breast surgical oncologist in the United States that offers the same variety of oncoplastic options, nor has contributed more to the different fields of breast reconstruction than Dr. Schwartz.