Fax: 770-339-9804
Lawrenceville, Georgia 30046


Originally published on PRS Global Open website in November 2021
Abstract
Summary:
The muscle-sparing latissimus dorsi flap relies on perforators from the descending branch of the thoracodorsal artery. Previous descriptions placed a transverse skin island independent of perforator location, as any design was thought to capture enough perforators to ensure flap survival. We have found this approach prone to complications when attempting breast reconstruction in obese patients who require large flap volumes. Although the most proximal perforators have the most reliable blood flow, inclusion of these perforators reduces the arc of rotation, as the flap would be close to the pivot point at the most cranial point of muscle division, leaving significant volume in the axilla. Here we describe a modified skin incision that includes all of the proximal perforators but also allows us free design of the skin island to harvest those areas of the back with maximal subcutaneous tissue and with enough distance from the pivot point to optimize arc of rotation.
Takeaways
Question: How can we increase the blood flow, rotational arc, and volume of the muscle-sparing latissimus dorsi flap for breast reconstruction in obese patients?
Findings: Inclusion of a strip of muscle, fat, and skin overlying the most proximal perforators of the latissimus muscle in continuity with a more distally placed flap improves outcomes in obese patients undergoing breast reconstruction.
Meaning: Improvement in blood flow, rotational arc, and flap volume can be achieved with a modified skin incision for the muscle-sparing latissimus dorsi flap, which is especially relevant in obese patients with large flap volumes.
INTRODUCTION
The muscle-sparing latissimus dorsi (MSLD) flap is a less invasive approach for breast reconstruction.1–4 This flap includes the descending branch of the thoracodorsal artery (TDA), which provides perforators for blood flow. The MSLD skin incision has been described as independent of perforator location as multiple perforators arise from the TDA beginning 6–10 cm from the posterior axillary fold.1–4 As such, any flap design of reasonable width will capture some perforators and dissection is unnecessary. Although certainly valid for most patients, the morbidly obese (Fig. 1) require large flap volumes for total breast reconstruction,5 and sufficient vascularization may not be present without inclusion of the most proximal, robust perforators. These perforators, however, are located close to the pivot point, which would make flap positioning challenging, as the arc of rotation is limited. Here, we describe a modified skin incision that includes the proximal perforators but positions the bulk of the flap inferiorly, providing a favorable arc of rotation. This results in an additional vertical scar in the posterior axillary line, which is inconspicuous in the obese.
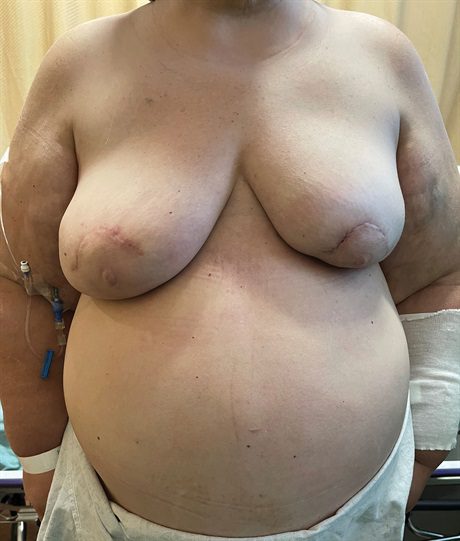 Fig. 1.:
Fig. 1.:
A 62-year-old morbidly obese (BMI = 47.6 kg/m2) woman after bilateral mastectomy and immediate direct-to-implant (790 ml) reconstruction for multicentric left breast cancer. Postoperatively, she has persistent dehiscence of the left periareolar mastectomy incision with serous drainage despite two attempts at implant salvage. We decide to convert her to an autologous reconstruction using the MSLD flap. She is a poor candidate for abdominal free tissue transfer given her morbid obesity and history of multiple abdominal surgeries. Her reconstructed left breast is smaller despite using the largest implant available.
METHODS
Morbidly obese patients [body mass index (BMI) ≥ 40 kg/m2] undergoing postmastectomy reconstruction (Fig. 1) were placed in the lateral decubitus position. Perforators were identified along the anterior edge of the latissimus dorsi (LD) with a handheld 8 MHz unidirectional Doppler. Transverse skin paddles were designed to harvest areas of abundant fat and to facilitate rotation into the breast regardless of perforator location.1–4 After definitive surgical identification of the anterior edge of the LD and confirmation that the preoperatively identified perforators were in the appropriate location, we committed to our superior incision with a generous cuff of additional skin and fat overlying the anterior edge of the LD to incorporate the most proximal musculocutaneous and septocutaneous perforators6 (Fig. 2). This adds a scar in the posterior axillary line (Fig. 3) but does not affect the final reconstructed breast shape (Fig. 4). The flap was deepithelialized, leaving a dermal bridge between the additional cuff of tissue overlying the proximal perforators and the body of the flap. (See Video [online], which demonstrates the preoperative markings and intraoperative dissection of a muscle-sparing latissimus dorsi flap with modified incision. This modification allows for inclusion of the proximal perforators to optimize blood flow and positioning of the flap independent of perforator location for improved arc of rotation and increased flap volume.) The remainder of the dissection was performed as previously described.1–4 Flap tissue oxygenation saturation was determined via near infrared spectroscopy using SnapshotNIR (Kent Imaging, Calgary, AB, Canada) and debrided as necessary. The rates of fat and flap necrosis requiring surgical intervention were compared with a historical group of patients with BMI greater than 40 kg per m2 who underwent MSLD reconstruction with standard incisions.
Video.
Video 1 from “Modified Incision for Muscle-Sparing Latissimus Dorsi to Increase Flap Perfusion in the Morbidly Obese.” This video demonstrates the preoperative markings and intraoperative dissection of a muscle-sparing latissimus dorsi flap with modified incision. This modification allows for inclusion of the proximal perforators to optimize blood flow and positioning of the flap independent of perforator location for improved arc of rotation and increased flap volume.
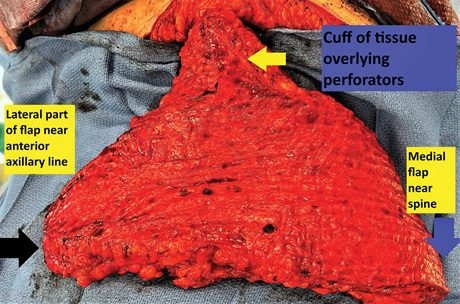 Fig. 2.:
Fig. 2.:
Intraoperative photograph of a 15 cm × 42 cm MSLD flap with extra cuff of dermis and fat overlying the proximal perforators (yellow arrow). The perforators are identified intraoperatively after we identify the anterior edge of the LD and design the cuff of tissue to include these perforators and any possible septocutaneus perforators located lateral to the muscle edge. The dermal connection between this cuff and body of the flap is kept intact to maximize collateral blood flow. The blue and black arrows denote the most medial and lateral extents of the flap, respectively.
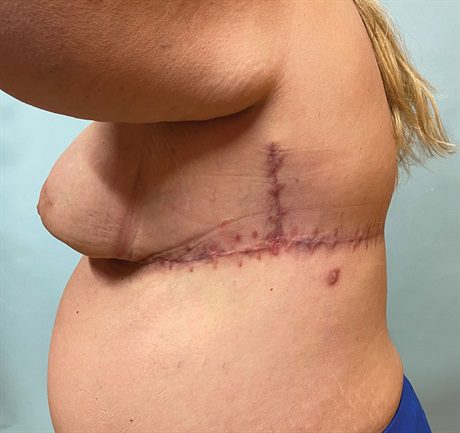 Fig. 3.:
Fig. 3.:
Postoperative donor site scarring demonstrating the extra vertical scar in the posterior axillary line where tissue is taken to include additional proximal perforators to the flap to increase blood flow. In these larger woman, this scar heals well and is relatively inconspicuous when their arms are by their sides.
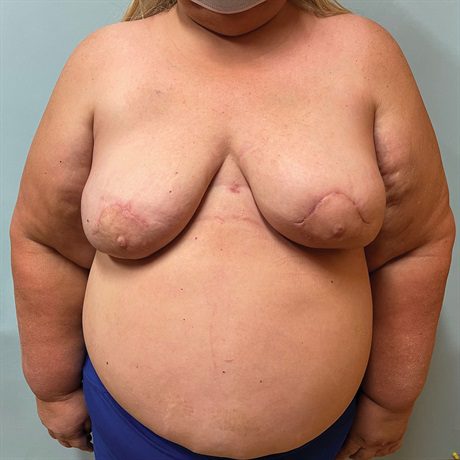 Fig. 4.:
Fig. 4.:
Four month postoperative result after MSLD reconstruction of the left breast. Her left mastectomy incision closed without incident. Her reconstructed left breast is now slightly larger than her right and therefore provides more volume than the largest prosthetics available. Given the prepectoral right breast implant reconstruction and its ptotic positioning, there is excellent symmetry with the purely autologous left breast reconstruction. This symmetry would not be possible with a right breast subpectoral implant reconstruction.
Statistical analyses were performed using IBM SPSS, version 23.0 (IBM Corp., Armonk, N.Y.). Continuous variables are presented as means and SDs and comparisons were made using independent Fisher exact test, whereas categorical variables were presented as frequency and proportions and compared using the chi-square test. Statistical significance was accepted as a P value less than 0.05.
RESULTS
Thirty-seven MSLDs in 25 patients were included with at least 6 months follow-up. The mean patient age was 56.2 years (range, 37–75 years), and BMI was 43.4 kg/m2 (range, 40.4–55.7 kg/m2). The average flap dimensions were 15.5 cm (range, 11–18 cm) by 38.2 cm (range, 31–50 cm). Eight (40%) patients were diabetics and fifteen (60%) were hypertensive.
Six (16.2%) flaps with modified MSLD incisions had clinically evident fat necrosis, whereas 20 (69.0%) flaps with standard incisions had fat necrosis clinically; the difference was significant (P = 0.00004). (See Table, Supplemental Digital Content 1,https://links.lww.com/PRSGO/B838.) One flap (2.7%) with the modified MSLD incision required surgical debridement, whereas seven (24.7%) flaps with standard incisions required surgical debridement of a portion of a necrotic flap; the difference was significant (P = 0.02). There were no statistically significant differences between patients who underwent modified MSLD incision versus standard incisions with respect to age, BMI, diabetes, hypertension, or flap dimensions (See Table, Supplemental Digital Content 1,https://links.lww.com/PRSGO/B838).
DISCUSSION
The MSLD with a transverse skin island for breast reconstruction was described without explicit perforator identification, relying on a 4–7 cm strip of LD for blood flow.1–4 In most clinical scenarios, this strategy works well and simplifies dissection. Morbidly obese patients have flap volumes significantly larger than the average patient, likely requiring more blood flow. As our experience with the MSLD grew, we realized that a large percentage of patients in the highest BMI ranges had clinically evident fat necrosis and even flap necrosis requiring debridement (unpublished results).
The most reliable perforators arise from the descending branch of the TDA, with the most proximal perforator often being dominant.1–4 This perforator may be septocutaneous or musculocutaneous or both.6 These perforators were not previously routinely included in our flaps as they lay close to the pivot point and restricted the arc of rotation. We felt that inclusion of these proximal perforators was important for blood flow to our largest flaps. We therefore modified our incision to include these perforators by keeping the overlying fat and dermis intact and in direct continuity with the remainder of the flap. This created an additional short scar in the posterior axillary line that was relatively inconspicuous in these women.
The MSLD has similar minimally associated postoperative functional disability and donor site complications as a TDAP flap, but is quicker, simpler, and safer to dissect, as no intramuscular dissection of the perforator is required and it does not require microvascular skills.1 In addition, the MSLD includes multiple smaller perforators that come along with the associated muscle segment, improving blood flow, which would be very technically difficult to include in a TDAP flap.7 This has presumably allowed us to describe here some of the largest flaps published for either an MSLD or TDAP flap by including these minor perforators and all the major perforators along the descending branch of the TDA.8 In our experience, the largest MSLD flaps in obese patients require this additional blood flow.
Saint-Cyr et al recommended maximizing adipofascial tissue harvest over the pedicle superior to the skin island to increase perfusion by including as many perforators as possible.9 Although this approach may increase blood flow, it increases dead space, which may increase seroma rates, devascularizes the skin (which may result in wound healing complications), and may leave contour deformities. In addition, collateral blood flow from the perforator to the remainder of the flap arises from direct and indirect connecting vessels in the superficial adipose tissues and subdermal plexus, respectively.10 We feel that the only reliable way to incorporate all of these connecting vessels is to transfer a full thickness segment of skin and fat overlying the perforators along with the underlying LD muscle.
CONCLUSIONS
MSLD reconstruction in obese patients requires reliable perfusion to prevent necrosis. We describe here a novel skin incision allowing us to include all the proximal perforators while also positioning the bulk of the flap distally, maximizing volume and arc of rotation.
REFERENCES
10. Schaverien M, Saint-Cyr M, Arbique G, et al. Three- and four-dimensional arterial and venous anatomies of the thoracodorsal artery perforator flap. Plast Reconstr Surg. 2008;121:1578–1587.
Jean-Claude Schwartz, MD, PhD – Breast Cancer and Reconstructive Surgeon

As a national leader in oncoplastic breast surgery, Dr. Schwartz has developed and published some of the most innovative ways to save women’s breasts, allowing them to avoid mastectomy and multiple reconstructive surgeries. Dr. Schwartz’s techniques have benefitted his patients and other surgeons around the world.
There is no breast surgical oncologist in the United States that offers the same variety of oncoplastic options, nor has contributed more to the different fields of breast reconstruction than Dr. Schwartz.