Fax: 770-339-9804
Lawrenceville, Georgia 30046


Originally published on PRS Global Open website in December 2015
Abstract
Summary:
Centers of excellence strive for high rates of breast conservation surgery. Given the increased patient satisfaction, evidence for improved survival, decreased rates of complications, reduced costs, and fewer surgical procedures compared to mastectomy and reconstruction, this makes sense. As such, surgeons have devised approaches to offer breast conservation to patients with more extensive disease that would have been classically recommended to undergo mastectomy. These ambitious attempts at breast conservation are supported by recent studies that have established their oncological safety. “Extreme oncoplasty” refers to Wise-pattern volume displacement surgery where the breast is immediately reconstructed after a multifocal or multicentric breast cancer is excised. The authors that described this concept also described a Wise-pattern “split reduction” to allow for excision of the skin directly over the cancer, insuring a clear anterior margin. Although extreme oncoplasty has been broadly discussed and published on by many groups, there are few reports that provide insight into the surgical details necessary to successfully perform this surgery. Here, we combine three different oncoplastic techniques: the Wise-pattern split reduction, immediate nipple reconstruction, and autologous volume replacement to demonstrate our approach to extreme oncoplastic breast conservation in a challenging patient.
INTRODUCTION
Oncoplastic breast conservation (OBC) allows surgeons to remove large breast cancers and immediately reconstruct the defect to avoid deformity. These ambitious attempts at breast conservation (BC) are warranted given recent data demonstrating a survival advantage for BC versus mastectomy1 and other studies documenting its oncological safety for multicentric breast cancers.2,3 BC also allows most patients to retain normal breast skin and nipple sensation; typically requires one surgery; and has fewer complications, reduced cost, and better patient reported outcomes4 compared to mastectomy and reconstruction. Given these findings, it behooves surgeons to innovate approaches that extend the indications for BC to patients with more extensive cancers.
Silverstein and Savalia coined the termed “extreme oncoplasty” where a Wise-pattern reduction was used to excise multifocal or multicentric cancers that were classically recommended to undergo mastectomy.5 In addition, they described the “split reduction,” which allowed for resection of the anterior skin margin, shifting the standard skin excision from the inferior pole of the breast to the breast mound and resulting in a modified Wise-pattern closure.6 While these strategies are useful, many patients with extensive breast cancers still have a volume deficiency after Wise-pattern closure and often require removal of their nipple-areolar complex (NAC), requiring additional strategies for successful BC. Several authors have described combining NAC reconstruction and Wise-pattern central lumpectomy7,8 and the author here published the first description of combining Wise-pattern volume displacement and autologous volume replacement to reconstruct larger defects in smaller, ptotic breasts.9 Here, we present a patient in whom these three different oncoplastic techniques are combined in one surgery to facilitate extreme OBC.
CASE
A 64-year old woman presented to our practice with a 7.2-cm left breast cancer that had not responded to neoadjuvant treatment (Fig. 1). Her lymph nodes were radiographically benign and her metastatic work-up was negative. Her tumor involved her NAC and upper outer quadrant skin, requiring sacrifice of both (Fig. 1). The patient preferred BC and was eager to avoid the complications associated with radiation and postmastectomy reconstruction. Given her ptosis and skin involvement, we offered her a Wise-pattern split reduction.6 We planned on immediately reconstructing her nipple from excess skin along the medial vertical limb.7 Given her small breast size and the extent of her breast cancer, we felt she also required autologous volume replacement.9 We chose the muscle-sparing latissimus dorsi (MSLD) flap, as it supplies more volume than the intercostal artery perforator flaps, is less morbid than a conventional latissimus dorsi flap, and is easier to dissect than a thoracodorsal artery perforator flap. The patient started in the lateral decubitus position where the MSLD is raised as previously described.10 She was then returned supine where her 215 g lumpectomy specimen (which includes her NAC) was removed and sent for intraoperative gross pathological assessment, and lymph nodes were sent for permanent section. Her contralateral breast then underwent a Wise-pattern mastopexy which we tried to match when reconstructing the left breast. Her MSLD flap was then transferred into her left breast lumpectomy defect and reduced in size and shaped to match the right breast (Fig. 2). (See figure, Supplemental Digital Content 1, which displays the intraoperative photograph demonstrating the MSLD flap filling the partial mastectomy defect. The modified Wise-pattern split reduction pattern is also shown with the lateral inframammary fold scar shifted superiorly to facilitate a clear anterior margin over the tumor. The CV flap is also demonstrated along the medial vertical limb before it is assembled into a nipple, https://links.lww.com/PRSGO/C746.)
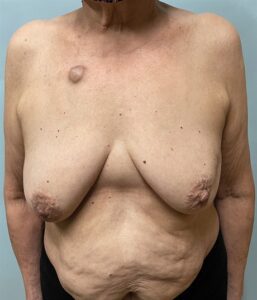 Fig. 1.:
Fig. 1.:
The patient, a 64-year-old woman with a 7.2-cm left breast cancer involving her left nipple and upper outer quadrant skin. She has grade 2 ptosis and Wise-pattern volume displacement will help facilitate her reconstruction. We will use a split reduction approach to resect her upper outer quadrant breast skin to insure a clear anterior margin. Given her small breast size, we predict that she will not have enough residual breast tissue to reconstruct her breast and plan on volume replacement with a muscle-sparing latissimus dorsi flap. We also plan on an immediate nipple reconstruction using excess skin in continuity with her medial vertical limb.
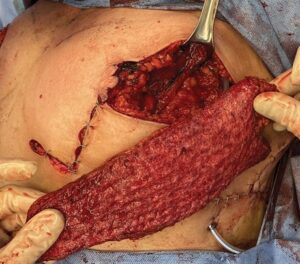 Fig. 2.:
Fig. 2.:
The patient’s partial mastectomy has been performed, and her defect in the upper outer quadrant is demonstrated. Her Wise-pattern split reduction is partially closed. The muscle-sparing latissimus dorsi flap, shown between the surgeon’s fingers, is used to fill her partial mastectomy defect. The excess skin that will be used for her CV flap nipple reconstruction is seen along her medial vertical limb.
Her left nipple was reconstructed with a CV flap off the medial vertical limb (Figs. 2 and 3). The Wise-pattern split reduction was then closed, and the areola reconstructed (Fig. 3). Her final pathology revealed widely clear margins and negative lymph nodes. She healed without incident and completed radiotherapy. She is shown 6 months after she completed radiotherapy (Fig. 4) with a well-healed donor site. (See figure, Supplemental Digital Content 2, which displays the well-healed donor site with scar in the posterior bra line 12 months after surgery, https://links.lww.com/PRSGO/C747). Patient satisfaction was assessed using a five-point Likert scale 12 months postoperatively (1 = strongly disagree, 2 = disagree, 3 = undecided, 4 = agree, 5 = strongly agree) for the following:
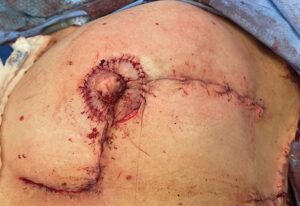 Fig. 3.:
Fig. 3.:
The patient’s left breast has been reconstructed with a Wise-pattern split reduction, immediate CV flap nipple reconstruction and muscle-sparing latissimus flap. The areola is fashioned by partially de-epithelializing the skin around the reconstructed nipple and then sewing it back down with a running baseball stitch.
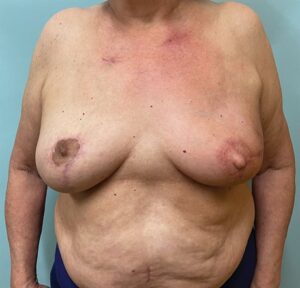 Fig. 4.:
Fig. 4.:
The patient is shown 6 months after the completion of radiotherapy. There has been some contraction and volume loss of her left breast, but she still maintains a good shape without deformity. She requires no further surgery but is planning on undergoing an areolar tattoo. Her successful outcome was dependent on incorporating the Wise-pattern split reduction, immediate nipple reconstruction and autologous volume replacement into one surgical procedure facilitating a successful oncological and reconstructive result.
She responded 5, 4, and 5 for the above questions, respectively.
DISCUSSION
For patients with ptosis, Wise-pattern volume displacement surgery provides the most flexibility and opportunity for repairing extensive breast defects. Silverstein and Savalia coined the termed extreme oncoplasty, and this approach was utilized in patients with multifocal or multicentric cancers that were classically recommended to undergo mastectomy.1 In addition, they innovated the oncoplastic split reduction, which allowed for a modified Wise-pattern closure and resection of the anterior skin margin, shifting a scar from the inframammary fold to the breast mound to guarantee a clear anterior margin. Although these contributions are important, we do not feel they adequately define or make clear to surgeons what this unique type of surgery often entails.
The purpose of this article was to give tangible meaning to the term extreme oncoplasty and insight into how this surgery can be practically performed. In this case report, we combine the Wise-pattern split reduction with MSLD volume replacement and immediate nipple reconstruction, giving surgeons a real-world example of how these difficult procedures are approached. Each of these procedures are unique and require a custom-made approach that may require the utilization and combination of different techniques for success. We feel that the future of extreme OBC will involve combining varied techniques in breast reconstruction, which might include the use of implants, acellular dermal matrix, fat transfer, combining local chest wall perforator flaps, free flaps, and other approaches that were not classically considered to be strategies necessary for BC surgery. We are hopeful that this report encourages other surgeons to propose new and creative approaches to solve the most challenging cases of extreme OBC.
CONCLUSIONS
Given the improved survival for patients undergoing BC compared to mastectomy and its proven safety in patients with multicentric breast cancers, oncoplastic surgeons must continue to innovate strategies to offer BC to women with extensive breast cancers. Although the concept of extreme oncoplasty is a useful one, the technical details necessary to perform this unique surgery have not been clearly documented in the literature. Here, we demonstrate a unique case of extreme OBC, combining autologous volume replacement and split Wise-pattern volume displacement surgery with immediate nipple reconstruction, providing a single example of the operative complexity necessary to perform these procedures. The future of extreme OBC will rely on the continued creativity of surgeons to combine varied techniques in breast reconstruction to provide patients the opportunity to save their breasts.
DISCLOSURE
The author has no financial interest to declare in relation to the content of this article.
REFERENCES
Jean-Claude Schwartz, MD, PhD – Breast Cancer and Reconstructive Surgeon

As a national leader in oncoplastic breast surgery, Dr. Schwartz has developed and published some of the most innovative ways to save women’s breasts, allowing them to avoid mastectomy and multiple reconstructive surgeries. Dr. Schwartz’s techniques have benefitted his patients and other surgeons around the world.
There is no breast surgical oncologist in the United States that offers the same variety of oncoplastic options, nor has contributed more to the different fields of breast reconstruction than Dr. Schwartz.