Fax: 770-339-9804
Lawrenceville, Georgia 30046


Originally published on PRS Global Open website in December 2015
Abstract
Background:
Oncoplastic breast conservation has been classically divided into volume displacement (VD) or volume replacement (VR) techniques. There have been few descriptions of merging these two approaches. This report describes our experience combining Wise-pattern VD and autologous VR to repair extensive partial mastectomy defects in patients with ptosis.
Methods:
A retrospective chart review was performed for patients who underwent combined Wise-pattern VD surgery and autologous VR by the author from June 2017 to June 2023, with at least 6 months follow-up. Patient demographics, oncological and intraoperative details, and complications were recorded.
Results:
Forty patients underwent Wise-pattern VD surgery combined with a medial intercostal artery perforator flap (five patients), lateral thoracic artery perforator/lateral intercostal artery perforator flap (18 patients), anterior intercostal artery perforator flap (five patients), or muscle-sparing latissimus dorsi flap (12 patients). The average tumor size was 4.0 cm (range, 1.5–9.1 cm), and specimen weight was 152 g (range, 33–415 g). Six patients (15%) required re-operation for positive margins. There was delayed healing of three (7.5%) donor sites. There were no flap failures. Two (5%) patients had clinically apparent fat necrosis without requirement for surgical revision.
Conclusions:
This report demonstrates the feasibility of combining Wise-pattern VD and autologous VR. We propose that oncoplastic breast-conserving surgery be no longer divided into two mutually exclusive approaches and that surgeons make liberal use of combining these approaches to address challenging cases of breast-conserving surgery.
Takeaways
Question: Can oncoplastic volume replacement (VR) and volume displacement be used together in one surgical procedure to facilitate oncoplastic breast conservation?
Findings: Oncoplastic VR and displacement can be safely used together for breast conservation.
Meaning: The merger of VR with a number of different perforator flaps and volume displacement in one surgical procedure may allow for surgeons to extend the benefits of breast conservation to a greater proportion of women who previously required a mastectomy.
INTRODUCTION
Oncoplastic breast-conserving surgery synthesizes both oncological and plastic surgery principles.1 This facilitates both complete resection of the malignancy and immediate reconstruction of the breast to avoid deformity, often with contralateral surgery for symmetry. This is critical in patients who almost all will undergo radiotherapy, because correcting defects afterwards is difficult, fraught with a higher risk of complication, and leads to poor aesthetic outcomes.2 There is a classical division in oncoplastic approaches described: those that involve a redistribution of residual breast tissue after oncological resection [volume displacement (VD)] versus those that describe transfer of nonbreast tissue from a local or distant site to immediately reconstruct the breast [volume replacement (VR)].3,4 Although some groups have described the use of breast implants as a VR strategy for oncoplastic breast-conserving surgery,5,6 this has been limited to smaller breasts with minimal ptosis. Most would agree that this approach is unproven and that placing an implant into a breast that will undergo radiotherapy has associated risk (capsular contracture and implant exposure).7,8 On the other hand, there is significant data demonstrating the durability of autologous tissue replacement to withstand the effects of adjuvant radiotherapy after partial mastectomy.9–11
There are patients with tissue deficits that will obviously require either a VR or VD strategy. Patients with small breasts, grade 0 ptosis and large defects will require VR to avoid a mastectomy or deformity. Patients with gigantomastia and grade 3 ptosis are more appropriate candidates for Wise-pattern VD. However, many patients are candidates for either a VD or VR approach. Most patients in the age range at risk to develop breast cancer have some degree of ptosis, which can be capitalized on to aid in reconstructing their partial mastectomy defect. The final decision on the surgical approach will depend on the patient’s desire to maintain her current breast volume, her acceptance of donor site scars and possible donor site complications, her willingness to undergo surgery on the contralateral breast, and the experience of the reconstructive surgeon.
In our experience, there is a group of patients who benefit from combining both autologous VR and wise-pattern VD to avoid mastectomy or deformity, to maintain breast volume, or simply to obtain the best aesthetic result possible. In 2018, the author published the first description of combining autologous VR and Wise-pattern VD for challenging cases of oncoplastic breast-conserving surgery.12 That report described combining a Wise-pattern mastopexy and an extended lateral intercostal artery perforator (LICAP) flap to harvest tissue from the back to reconstruct defects regardless of their breast location. We have since abandoned the extended LICAP flap for the muscle-sparing latissimus dorsi (MSLD) flap, which has superior blood flow, when such large volumes are required for VR.13,14 In addition, we have refined our approach to VR as we now combine the Wise-pattern mastopexy with different local chest wall perforator flaps [anterior intercostal artery perforator (AICAP), medial intercostal artery perforator (MICAP), and lateral intercostal artery perforator (LICAP)/lateral thoracic artery perforator (LTAP)] depending on the location and size of the breast defect. In this report, we demonstrate our experience with combining wise-pattern VD and autologous VR to facilitate oncoplastic breast-conserving surgery in ptotic patients where either approach alone was not sufficient.
PATIENTS AND METHODS
After institutional review board approval, we retrospectively reviewed the charts of patients who underwent breast conservation using a Wise-pattern mastopexy and simultaneous autologous VR using an AICAP (Fig. 1, Video 1), LICAP/LTAP (Fig. 2, Video 2), MICAP (Fig. 3, Video 3) or MSLD flap (Fig. 4, Video 4) from June 2017 through June 2023 with at least 6-month follow-up. [See Video 1 (online), which demonstrates the use of the anterior intercostal artery perforator flap in combination with a superior pedicle Wise-pattern mastopexy to reconstruct an inferior-central breast defect. Preoperative and postoperative results are presented in Fig. 4]. [See Video 2 (online), which demonstrates the use of a lateral intercostal artery perforator flap in combination with a Wise-pattern closure to reconstruct a central defect involving the nipple. She undergoes immediate nipple reconstruction and contralateral mastopexy for symmetry.] [See Video 3 (online), which demonstrates the medial intercostal artery perforator flap in combination with a Wise-pattern mastopexy being using to reconstruct an inferior breast defect. Preoperative and postoperative results are demonstrated in Fig. 1.] [See Video 4 (online), which demonstrates two patients undergoing MSLD reconstruction in combination with an immediate Wise-pattern mastopexy. The contralateral breasts undergo mastopexy for symmetry. The first patient undergoes a delayed reconstruction after confirmation of clear margins a week prior, whereas the second patient undergoes immediate MSLD reconstruction of her extensive deficit. We very selectively perform immediate MSLD reconstructions after partial mastectomy, as we favor confirming clear margins first.]
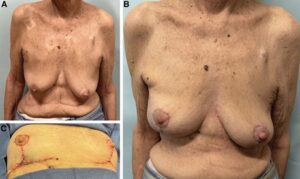 Fig. 1.:
Fig. 1.:
Wise-pattern VD in combination with an AICAP flap. A 66-year-old woman with a multifocal right breast cancer spanning 4 cm (A). She undergoes a right partial mastectomy and reconstruction with an anterior intercostal artery perforator flap and superior pedicle mastopexy (Video 1). Her on-table result (B) demonstrates good symmetry without deformity. She is shown 6 months after partial breast radiotherapy (C) with elevation of the reconstructed breast but with no evidence of breast deformity.
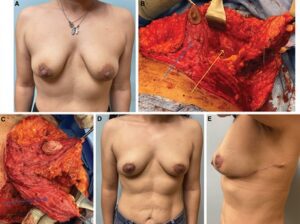 Fig. 2.:
Fig. 2.:
Wise-pattern VD in combination with an LICAP flap. A 44-year-old woman with a 3-cm upper outer quadrant left breast cancer (A). Her left partial mastectomy (black arrow) leaves her with a significant defect (yellow arrow). We combine an inferior pedicle (blue arrow) mastopexy (B) and lateral intercostal artery perforator flap (blue arrow) to reconstruct her defect (C) (Video 2). She is shown 6 months after the completion of radiotherapy (D), with a well-healed donor site (E).
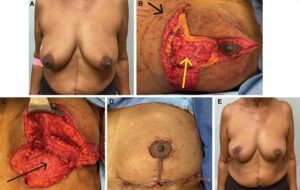 Fig. 3.:
Fig. 3.:
Wise-pattern VD in combination with an MICAP flap. A 65-year-old woman with three foci of left breast cancer between 5 and 7 o’clock spanning 5 cm (A). Preoperatively, we identify two strong medial intercostal artery perforators (black arrow). The volume of the flap is located below the inframammary fold (red arrow). The partial mastectomy specimen (yellow arrow) is localized by radiology before surgery with three wires (blue arrows) (B). The defect is reconstructed with a medial intercostal artery perforator flap (black arrow) and Wise-pattern mastopexy (C, D) (Video 3). Eighteen-month follow-up result demonstrates no evidence of deformity (E).
Video 1.
This video demonstrates the use of the anterior intercostal artery perforator flap in combination with a superior pedicle Wise-pattern mastopexy to reconstruct an inferior-central breast defect. Preoperative and postoperative results are presented in Figure 4.
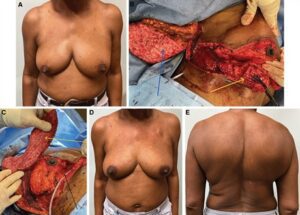 Fig. 4.:
Fig. 4.:
Wise-pattern VD in combination with an MSLD flap. A 54-year-old woman with a multifocal right breast cancer at 6 o’clock (A). She undergoes a superomedial Wise-pattern mastopexy and VR using an MSLD flap. The right partial mastectomy (yellow arrow) and muscle-sparing latissimus flap (blue arrow) are shown (B). The partial mastectomy defect (black arrow), extended superomedial pedicle (blue arrow), and muscle-sparing latissimus flap (yellow arrow) are shown after the cancer is removed (Video 4). The entire flap is used to reconstruct the defect (C). The patient is shown 8 months after the completion of radiotherapy with no evidence of deformity (D) and a well-healed donor site (E).
Video 2.
This video demonstrates the use of a lateral intercostal artery perforator flap in combination with a Wise-pattern closure to reconstruct a central defect involving the nipple. She undergoes immediate nipple reconstruction and contralateral mastopexy for symmetry.
Video 3.
This video demonstrates the medial intercostal artery perforator flap in combination with a Wise-pattern mastopexy being using to reconstruct an inferior breast defect. Preoperative and postoperative results are demonstrated in Figure 1.
Video 4.
This video demonstrates two patients undergoing MSLD reconstruction in combination with a immediate with a Wise-pattern mastopexy. The contralateral breasts undergo mastopexy for symmetry. The first patient undergoes a delayed reconstruction after confirmation of clear margins a week prior while the second patient undergoes immediate MSLD reconstruction of her extensive deficit. We very selectively perform immediate MSLD reconstructions after partial mastectomy as we favor confirming clear margins first.
Previously reported patients who underwent Wise-pattern VD and extended LICAP VR were excluded from this study.12 This flap was replaced with the MSLD, as our experience with the extended LICAP demonstrated a significant rate of delayed fat necrosis (unpublished data). No patients in this series underwent VR with an extended LICAP flap. LICAP/LTAP flaps included here were designed as previously described as turnover flaps, including a “mesentery” of perforators coming from both the LICAP and LTAP vessels.9,10 Active smokers were excluded from this study unless they abstained from nicotine products for 30 days before and after surgery. All patients underwent immediate contralateral mastopexy/reduction. All procedures, including both the oncological resection and reconstruction, were performed by the author at an outpatient ambulatory surgery center.
The following patient variables, and operative and oncological details were recorded: age, body mass index, patient comorbidities (diabetes, smoking, hypertension), size of malignancy, requirement for neoadjuvant or adjuvant chemotherapy, preoperative cup size, breast ptosis, specimen weight, type of flap and nipple pedicle utilized, operative duration, major and minor complications, rate of clinical flap fat necrosis (defined a palpable hardening in the reconstructed breast with at least 6 months follow-up), and length of follow-up. All statistical analyses were performed using IBM SPSS, version 25.0 (IBM Corp., Armonk, N.Y.).
PREOPERATIVE MARKINGS AND OPERATIVE TECHNIQUE
Patients were marked in the standing position with the Wise-pattern. All patients had undergone wire localization of their breast cancers and injection of technetium to facilitate sentinel lymph node biopsy. In some patients, a previously biopsied axillary lymph node that was positive for metastatic carcinoma was also wire-localized by radiology. Chest wall perforator flaps were designed beneath the inframammary fold (IMF) for MICAP and AICAP flaps and lateral to the breast footprint for LICAP/LTAP flaps, as previously described.10,15,16 MSLD flaps were designed as transverse skin islands that extended to the posterior spine, as previously described.13,14,17 To minimize position changes for patients undergoing immediate MSLD reconstruction, patients started in the lateral decubitus position for flap dissection and were then returned supine for the cancer resection and reconstruction. After the cancer resection and lymph node dissection were completed, a contralateral mastopexy was performed preserving all breast volume, providing a shape and size to match for the reconstructed breast. For flaps other than the MSLD, we typically replace just the volume removed (and performed a mastopexy), giving us a good match with the nonpathological breast. This is similar to traditional VR surgery, where the volume resected is simply replaced without modification of the contralateral breast. Here, we are also adding a mastopexy to both sides. This is a bit different for MSLD reconstructions, as the volume of the MSLD is sometimes far greater than needed to replace the volume removed. Here, we still perform a mastopexy on the nonpathological breast first and then proceed to shape the flap and diseased breast (sometimes removing tissue from the flap or breast or both) to give us the best match possible (Video 4). We rarely need to modify or re-open the nondiseased breast after we have reconstructed the diseased breast.
The defect was then evaluated, and the nipple pedicle decided upon. For upper-inner and lower-inner breast defects, MICAP flaps were used. For inferior-central defects, MICAP or AICAP flaps were used. When defects could be addressed by multiple flaps, the best Doppler signals confirming a reliable perforator dictated our flap choice. Lateral defects were addressed with LICAP/LTAP flaps. For the very largest defects (including the most challenging upper-inner quadrant defects), MSLD flaps were planned. Very often, MSLD flaps were performed using a delayed-immediate approach where the cancer was resected, no drain is placed, and the cavity is filled with water. This keeps the skin envelope open, preventing retraction. Water can be injected into the breast in the office if there is a delay in reconstructing the breast and water volume is decreasing. Water persists longer than saline for unclear reasons (Stephen McCulley, personal communication). This approach is useful when one wants to confirm clear margins before committing to a flap based off the thoracodorsal vessels in a second surgery.
After confirming clear margins, a second surgery was planned to complete the mastopexy and MSLD reconstruction and contralateral mammoplasty. For MICAP, AICAP, LICAP/LTAP flaps, closing the donor site and/or reconstructing the IMF was performed before insetting the flap. The flap was then loosely sutured to the surrounding breast tissue, and Wise-pattern mastopexy performed (Videos 1–4). At this point, final adjustments were made by either reducing the contralateral breast or adjusting the flap volume (most commonly with MSLD flaps which can augment the breast to a larger size than the native contralateral breast). All flaps were debrided (if necessary) and demonstrated punctate arterial bleeding at their distal edge before they were inset. Drains were placed into all reconstructed breasts and MSLD, LICAP/LTAP donor sites. Patients were discharged home and seen on postoperative day six. All patients subsequently went on to adjuvant whole breast radiotherapy (with intervening chemotherapy if required).
RESULTS
The mean patient age, body mass index, and follow-up was 55.3 years (range, 36–76 years), 27.6 kg/m2 (range, 19–43kg/m2), and 13.6 months (range, 6–60 months), respectively. Five (12.5%) and ten (25%) percent of patients had a formal diagnosis of diabetes mellitus and hypertension, respectively. (See table, Supplemental Digital Content 1, which displays the demographics, operative details, and oncological characteristics of patients undergoing combined Wise-pattern mastopexy and autologous flap reconstruction. https://links.lww.com/PRSGO/D130.) Ten (25%) patients were former smokers, and four (10%) had agreed to abstain for 30 days perioperatively. Breast cancer staging was recorded as stage 0 (9), stage 1 (5), stage 2 (6), stage 3 (19), and stage 4 (1). The mean operative time for non-MSLD VR surgery was 115 minutes (range, 93–182 min). The mean operative time for combining immediate MSLD VR and Wise-pattern mastopexy with contralateral symmetry surgery was 183 minutes (range, 157–236 min). Delayed MSLD and Wise-pattern mastopexy with contralateral symmetry surgery averaged 170 minutes (range, 140–200 min). Neoadjuvant chemotherapy and adjuvant chemotherapy were delivered to 20% and 25% of patients, respectively. The average tumor size was 4.0 cm (range, 1.5–9.1 cm), and specimen weight was 152 g (range, 33–415 g). Most patients had grade 1 (27.5%) or grade 2 breast ptosis (65%), and a few patients had grade 3 ptosis (7.5%). Preoperative cup sizes were estimated and documented in Supplemental Digital Content 1, which showed that most patients undergoing MICAP or AICAP flaps had B or C cup breasts, and patients undergoing LICAP/LTAP or MSLD reconstructions had C or D cup breasts (the average specimen weight for each flap reconstruction is also documented). Two patients undergoing LICAP reconstruction, one patient undergoing MSLD reconstruction, and one patient undergoing MICAP reconstruction underwent resection and immediate reconstruction of their nipple and areola. Three patients underwent resection of skin over their tumor requiring a modified “split reduction” pattern.18,19 Six patients (15%) were found to have positive margins. Four of these patients were successfully re-excised, and two elected to proceed with completion mastectomy (not included in the cohort).
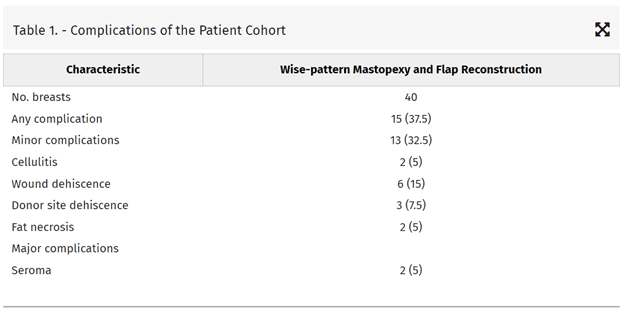
Complications are documented in Table 1. There were no incidences of nipple necrosis or flap failure. Six (15%) reconstructed breasts developed T-junction dehiscence, all of which healed within 6 weeks with local wound care. There were two (2.5%) incidences of presumptive breast cellulitis that resolved with a 7-day course of oral antibiotics. There were no incidences of abscess or hematoma. All MICAP and AICAP flap donor sites healed without complication. There was one (7.1%) LICAP/LTAP donor site dehiscence and one (7.1%) that developed a persistent seroma requiring re-operation for drain placement. There were two (16.7%) MSLD donor sites that dehisced and one (8.3%) that developed a persistent seroma requiring re-operation. The MSLD donor site dehiscences were treated with wound vac therapy and healed within 8 weeks. Although several patients had palpable areas in their reconstructed breasts in proximity to where the flap was placed in the immediate postoperative period, all but two (MICAP and MSLD reconstructions; 5%) of the patients had resolution of these areas by 6 months (presumably fat necrosis).
DISCUSSION
Although we show in this report that ambitious attempts at breast conservation are technically feasible, for a long time it was unclear if breast-conserving surgery for extensive breast cancers was oncologically sound. Silverstein et al introduced the concept of “extreme oncoplasty,” which used Wise-pattern VD surgery to perform breast conservation in multifocal or multicentric breast cancers that were typically recommended to undergo mastectomy.18,20 Although he demonstrated acceptable cosmesis and local recurrence and survival rates, this was a single-institution and surgeon series, which may not be generalizable. Recently, however, there is objective clinical data that demonstrate equivalent local recurrence rates for patients with multiple ipsilateral breast tumors and those with unifocal cancers.21,22 These data, in combination with multiple recent studies that demonstrate a superior local recurrence and survival rate for breast conservation versus mastectomy,23 encourage surgeons to continue to push the boundaries of breast conservation.19
In our opinion, there has been an unnatural division between patients who are appropriate candidates for VD versus VR, as if they were mutually exclusive approaches. Women in the age range at risk to develop breast cancer usually have some ptosis that can be capitalized on to aid in reconstructing their breasts. Many of these women will still not have enough residual breast tissue after an extensive breast cancer is resected and would be offered a nipple-sparing mastectomy by many surgeons as neither a mastopexy nor VR alone would be sufficient for successful breast conservation. We believe these patients are better served by combining VR and VD to offer these women the benefits of breast conservation (retained breast and nipple skin sensation; fewer procedures; reduced cost, recovery time and complications; and improved satisfaction and survival).
In 2018, we published the first report describing combining a Wise-pattern mastopexy and autologous VR using an extended LICAP that recruited tissue from the back.12 Although this was effective in most patients with central or lateral breast defects (75% of all breast cancers), this approach was less optimal in patients with inferior and medial breast cancers. We also found a significant rate of delayed fat necrosis in these patients and believe the lateral intercostal perforators are likely not reliable to support these larger flaps. These flaps also lost a significant percentage of the subcutaneous tissue available for a future flap based off the thoracodorsal vessels, despite leaving the thoracodorsal artery and latissimus dorsi muscle undisturbed. Very often, this large amount of tissue was unnecessary in reconstructing a breast defect, which led us to begin to use local chest wall perforator flaps, which harvested less tissue and required less extensive surgery without an intraoperative position change. These local chest wall perforator flaps were especially useful for lower-inner, lower-central, and upper-inner quadrant breast defects. It should be noted that although the LICAP/LTAP and MSLD flaps are easily incorporated into Wise-patterns using any type of nipple pedicle, the MICAP, AICAP, and reverse LICAP24 (none included in this study) flaps, which come from below the IMF, cannot be used with inferior pedicles. These flaps interfere with blood flow to the inferior pedicle when they are both raised and mobilized, as transection of the posterior perforating vessels that supply the inferior pedicle is routinely necessary. MICAP, AICAP, and reverse LICAP flaps are best used with superior, medial, or lateral nipple pedicles, which make them perfect choices for reconstructing inferior breast defects.
It should be noted that not all patients in this series underwent extreme oncoplasty, and the approach described here is not limited to cancers that are so large that they challenge our previous standards of who is eligible for breast conservation. Several patients in this series had T1 tumors (<2 cm) but had such small breasts and or tumors located in difficult locations (inner and lower quadrants) that VD alone would not have sufficed for reconstruction. This approach is effective in patients with some degree of ptosis and a large tumor to breast size ratio, regardless of the absolute tumor or breast size.
These procedures are of longer duration than standard VD and VR procedures alone, especially when the MSLD flap is used, which requires a position change. In addition, we favor a delayed-immediate approach, requiring two procedures, for combining VD and the MSLD, as this allows us to precisely determine how much of the MSLD flap is required for VR after clear margins are confirmed. Immediate MSLD reconstruction exhausts a major reconstructive modality in a patient who may require mastectomy if pathology confirms more extensive disease than planned for preoperatively. Regardless, losing the latissimus dorsi muscle to conserve the breast might make some uneasy. However, local recurrence rates after partial mastectomy and radiation are low (approximately 5%–10%),21–23 and likely less than after mastectomy. Given the improved survival rates for breast conservation over mastectomy, the high risk of complication of radiating a postmastectomy reconstruction (most of these patients will require radiotherapy regardless of whether they undergo breast-conserving surgery or mastectomy) and the increasing acceptance of repeat breast conservation after a local recurrence in certain patient groups, I believe this approach is warranted. We would still recommend preferential use of a generous LICAP flap over the MSLD, if possible, to keep this lifeboat flap available in case of a future local recurrence.
Re-excision of positive margins can also be challenging as the flap adds additional complexity to the anatomy of the dissection. As the author performs both the oncological resection and reconstruction, this facilitates both scheduling and performing a re-excision. We have also found that going back within 2 weeks before the flap begins to incorporate into the breast tissue allows for easier identification of the lumpectomy cavity, which has been marked by titanium clips, and the relevant margins that require re-excision. Although the aesthetic outcomes of oncoplastic breast-conserving surgery and VR after radiotherapy have each been individually shown to be acceptable in long-term follow-up, the combination of these approaches, as presented in this study, still requires further study to demonstrate longer-term efficacy. These procedures also require additional familiarity with the anatomy and dissection of perforator flaps, for which not all reconstructive surgeons have the necessary training or experience.25
CONCLUSIONS
In this study, we demonstrate the feasibility of combining Wise-pattern VD and VR with several different perforator flaps to facilitate oncoplastic breast-conserving surgery. This new approach will allow reconstructive surgeons to extend to a great number of patients the benefits of breast conservation who previously required mastectomy or who had poor results with either VR or VD techniques alone.
DISCLOSURE
The author has no financial interest to declare in relation to the content of this article.
REFERENCES
Jean-Claude Schwartz, MD, PhD – Breast Cancer and Reconstructive Surgeon

As a national leader in oncoplastic breast surgery, Dr. Schwartz has developed and published some of the most innovative ways to save women’s breasts, allowing them to avoid mastectomy and multiple reconstructive surgeries. Dr. Schwartz’s techniques have benefitted his patients and other surgeons around the world.
There is no breast surgical oncologist in the United States that offers the same variety of oncoplastic options, nor has contributed more to the different fields of breast reconstruction than Dr. Schwartz.