Fax: 770-339-9804
Lawrenceville, Georgia 30046

Originally published on PRS Global Open website in June 2017
Reconstructive surgeons are encountering an increasing number of obese women requiring postmastectomy reconstruction. These patients are poor candidates for autologous and prosthetic-based reconstructions as they have a high rate of reconstructive failure, surgical complications, and poor aesthetic outcomes. We demonstrate here the utility of the previously described Goldilocks mastectomy with free nipple grafts as a safe bridge to second-stage implant-based breast reconstruction.
Ten consecutive morbidly (BMI > 40) or super obese (BMI>50) women underwent bilateral Goldilocks mastectomy with free nipple grafts followed by second-stage subpectoral implant placement at least three months postoperatively. Patients were assessed for implant-related complications including malposition, capsular contracture, dehiscence, and extrusion.
Ten postmastectomy reconstructions in patients with BMIs ranging from 37 to 50 with a mean BMI of 45 underwent bilateral Goldilocks mastectomy with free nipple grafts. Two patients had wound healing complications after Goldilocks mastectomy but were completely healed within 8 weeks. There were no instances of delayed wound healing or reconstructive failure after prosthetic placement. With at least 9 months of follow-up on all patients, no patient has had a capsular contracture, significant malposition, or other complication requiring reoperation.
The obese patient poses a significant reconstructive challenge for which no reproducible approach has been described. Here, we present a 2-stage strategy: the previously described Goldilocks mastectomy with free nipple grafts followed by second-stage subpectoral definitive implant placement. This is the first proposed description of a reliable strategy for postmastectomy reconstruction in the morbidly and super obese.
The increased rates of obesity coupled with the increased rate of breast cancer development in the obese has resulted in a patient population for whom there are few reconstructive options after mastectomy.1,2 Multiple studies have shown unacceptable complication rates for both prosthetic and autologous reconstruction in these patients.3–5 These complications include implant and flap loss and reconstructive failure, skin necrosis, wound complications, fat necrosis, and donor-site complications.
The previously described “Goldilocks mastectomy” was developed for patients who were poor candidates for traditional postmastectomy reconstruction.6 This technique involves a skin-sparing mastectomy through Wise incisions and utilizes the residual cutaneous mastectomy flaps to create a breast mound. We developed an updated version of this technique that includes a free nipple graft and aggressive sculpting of the inferior mastectomy flap with division of the lateral inframammary fold for medial tissue transfer to create a more central breast mound.7,8 In the minority of women with significant macromastia and ptosis, this might allow for a single-stage autologous reconstruction. Most women require additional volume supplementation, some of whom can be accommodated with lipotransfer surgery.9 However, most obese women require significant additional volume that must be supplied by an implant or flap. Here, we present successful implant-based reconstruction in 10 consecutive women who were either morbidly obese or super obese [body mass index (BMI), 37–50; average, 45]. In these women, Goldilocks mastectomy with free nipple grafts was followed by definitive submuscular implant placement 3 months later without an instance of reconstructive failure or significant complication that delayed care.
The technique for Goldilocks mastectomy with free nipple grafts is described in Figures 1, 2 as previously described.8 Representative obese preoperative patients are shown in Figure 3. In Figure 4, we demonstrate postoperative Goldilocks mastectomy patients 3 months after surgery. In Figure 5, we have 1-year follow-up after second-stage subpectoral definitive implant placement.
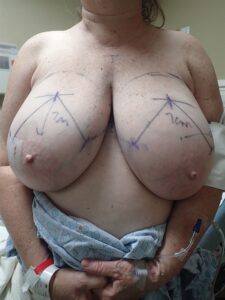 Fig. 1.:
Fig. 1.:
Representative obese woman with preoperative markings for a Wise Pattern bilateral mastectomy. Full thickness incisions are made through the lateral and medial extensions from the vertical limbs to the inframammary fold. These incisions are connected across the breast in preparation for mastectomy. We do not incise the vertical limbs as the involuted tissue between the limbs adds projection upon closure. The NACs are resized and saved for later grafting.
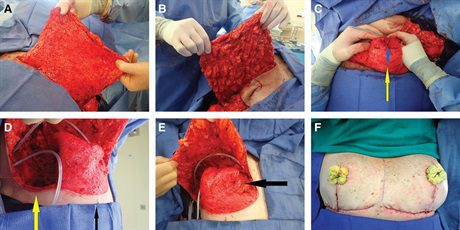 Fig. 2.:
Fig. 2.:
A, B The deepithelialized inferior mastectomy flap that forms the bulk of the reconstructed breast mound is shown. To maximize the amount of volume available for reconstructing a breast mound centered on the meridian, we find it useful to divide the dermis in the inframammary fold laterally, leaving two-thirds connected medially, allowing for medial tissue transfer (demonstrated in a subsequent panel). C, Demonstrates how we reconstruct the breast mound. The inferior dermal flap is folded in half along a transverse axis creating a double thickness flap. This folded flap is then folded again on itself along the vertical meridian (yellow arrow) creating 2 pillars. At this point, we use a 2-0 absorbable suture to stabilize the reconstructed breast mound by suturing the 2 pillars to each other at the base near the inframammary fold (IMF). We continue the interrupted suturing superiorly to the apex of the reconstructed mound (blue arrow demonstrates apical suture). We also suture this reconstructed mound to the pectoralis major muscle. We typically choose a point 6 to 7 cm above the IMF as the most superior point on the pectoralis, where we suture the mound in place. This will position the bulk of the tissue near the IMF, where we want it, to maximize lower pole fullness and nipple projection. D, Demonstrates the division of the inferior mastectomy flap in the lateral one-third at the IMF (yellow arrow). We then transfer this tissue at the lateral IMF as far medially as possible—up, over, and partially behind the reconstructed breast mound—and suture it to a parasternal location, which provides additional projection, height, and medial fullness. E, This transposition results in transfer of tissue from the most lateral point on the IMF to the most medial point in the newly reconstructed breast mound (black arrow at parasternal location was previously located at the far lateral IMF). F, The Wise flaps are closed over the breast mound with additional projection provided by the tissue between the vertical limbs, which is finally followed by NAC grafting. Note the asymmetry between the breasts that often results after a cancer resection (right) and prophylactic mastectomy (left).
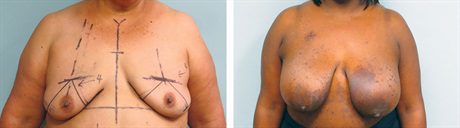 Fig. 3.:
Fig. 3.:
Representative high-risk preoperative cancer patients who will undergo bilateral mastectomy. The African American patient also has significant eczema, which makes immediate reconstruction unappealing.
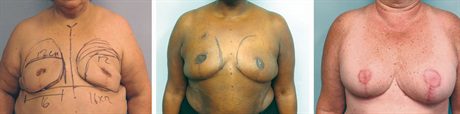 Fig. 4.:
Fig. 4.:
Three months postoperative after Goldilocks mastectomy with free nipple grafts. We have recruited as much inferolateral dermis and fat medially to create a breast mound, but these patients all desire additional volume supplementation. The last patient is the postoperative result from Figure 1. She has asymmetry between the breasts, which can be improved with implant placement.
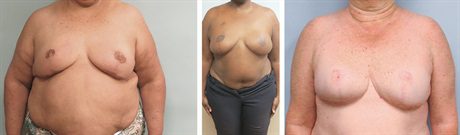 Fig. 5.:
Fig. 5.:
More than 1 year postoperative following second-stage subpectoral implant placement in all 3 patients. The last patient underwent just right implant placement with improvement of her asymmetry.
Obesity is a risk factor for complications after autologous and implant-based postmastectomy reconstruction. As these increased rates of complications are thought to be linearly related to increasing BMI,10 one would expect that the morbidly obese (BMI > 40) and super obese (BMI > 50) would have the highest rates of complication. Indeed, many reconstructive surgeons would certainly regard these patients as not appropriate candidates for breast reconstruction of any kind.
Here, we present 10 postmastectomy reconstructions in patient with BMIs ranging from 37 to 50 with a mean BMI of 45. All 10 patients had diagnosis of unilateral cancers but chose to undergo bilateral mastectomy. Two of 10 patients had wound healing complications after Goldilocks mastectomy and reconstruction but were completely healed within 8 weeks of their index surgery. All 10 patients underwent subpectoral breast reconstruction at a minimum of 3 months after their initial surgery. There were no implant-related complications, instances of delayed wound healing, or reconstructive failures after prosthetic placement. With at least 9 months of follow-up on all patients, we have no instances of capsular contracture, significant malposition, or other complications requiring reoperation.
There have been many theories on why increasing BMI results in increased rates of surgical complications including longer operative times, increased dead space with resultant seroma formation, poorer perfusion, and compromised wound healing. Our strategy presented here for successful implant-based reconstruction delays placement of prosthetic material into the postmastectomy milieu until there is complete healing of the mastectomy flaps. This minimizes risk of implant extrusion, infection, or malposition, which are at highest risk in the immediate setting when the flaps are ischemic and the tissue less reliably maintains the implant in place. This strategy effectively makes second-stage prosthetic placement nearly equivalent in safety and reliability to that of an elective subpectoral breast augmentation. In addition, we immediately salvage the nipple as a graft during the initial surgery, placing it into ideal position. In addition to insuring that the prosthetic is placed into a sterile and well-perfused environment, we manage the excess skin envelope in the initial surgery, deepithelializing and involuting the excess skin. This skin and underlying fat are used for soft-tissue coverage over the implant, centering this excess tissue over the meridian by recruiting it from the inferolateral mastectomy flap as previously described.8 This skin and soft-tissue rearrangement in the initial surgery allows for reasonable aesthetic outcomes in the obese using implant-based techniques. This is because we now have an ideal skin envelope and have shifted excess lateral skin and fat medially, which allows us to match a premade breast implant with a limited volume range to a more manageable space—the bony thorax plus the additional soft-tissue coverage supplied by the Goldilocks mastectomy. In this way, standard sized implants can be used to effectively and safely reconstruct the morbidly and super obese with very acceptable aesthetic outcomes in 2 simple surgeries.
The obese patient poses a significant postmastectomy reconstructive challenge for which no reproducible approach has been described. Here, we present a 2-stage strategy—the previously described Goldilocks mastectomy with free nipple grafts followed by second-stage subpectoral definitive implant placement. This strategy allows us to delay the mastectomy flaps before implant placement, salvage nipple-areola complex as a free nipple graft and establish a manageable soft-tissue envelope, where standard sized implants can be used to safely reconstruct patients with the very highest BMIs with good aesthetic outcomes.
We thank Dr. Piotr Skowronski for his assistance and thoughtful insight.
Jean-Claude Schwartz, MD, PhD – Breast Cancer and Reconstructive Surgeon

As a national leader in oncoplastic breast surgery, Dr. Schwartz has developed and published some of the most innovative ways to save women’s breasts, allowing them to avoid mastectomy and multiple reconstructive surgeries. Dr. Schwartz’s techniques have benefitted his patients and other surgeons around the world.
There is no breast surgical oncologist in the United States that offers the same variety of oncoplastic options, nor has contributed more to the different fields of breast reconstruction than Dr. Schwartz.