Fax: 770-339-9804
Lawrenceville, Georgia 30046


Originally published on PRS Global Open website in July 2020
Abstract
Background:
Mastectomy and implant-based reconstruction is typically performed in a hospital setting (HS) with overnight admission. The aim of this study was to evaluate postoperative complications and outcomes with same-day discharge from an ambulatory surgery center (ASC) compared with the same surgery performed in the HS.
Methods:
Patients who underwent mastectomy and immediate prepectoral tissue expander reconstruction were included in this retrospective study. Surgery was performed in an ASC with same-day discharge or the HS with overnight observation or same-day discharge. Patient demographics, operative details, outcomes, complications, and patient satisfaction were compared.
Results:
One hundred six women (183 breasts) underwent surgery in the HS, and 103 women (177 breasts) had their surgery in an ASC between August 2014 and September 2019. Demographics, comorbidities, and operative details were similar. Although there was no difference in the rates of most major complications, infectious complications requiring operative intervention were less frequent in the ASC [2.3% (n = 4) versus 11.5% (n = 21); P = 0.001]. Patient satisfaction, evaluated with a 5-point Likert scale, was higher in the ASC.
Conclusions:
Mastectomy and prepectoral reconstruction in an ASC is a safe alternative to the standard approach of performing this procedure in the HS. Although the rates of most surgical complications are similar between the HS and ASC, we have found a significantly reduced rate of major infectious complications requiring surgical intervention in the ASC which reduces overall cost and patient morbidity. Finally, patient satisfaction was higher in the ASC compared with the HS.
INTRODUCTION
Implant-based reconstruction after mastectomy represents >80% of the reconstructive procedures performed, with nearly 90% of these using a 2-stage approach with tissue expansion.1 The prepectoral approach has recently gained significant traction with an increasing number of prosthetic reconstructions performed in this manner.2 Several recent publications have documented equivalent complication rates and safety compared with the subpectoral approach even in the most challenging patients.3–7 The benefits of the prepectoral approach include less postoperative pain and quicker recovery, an easier and faster dissection, and elimination of postoperative animation deformity.
As surgical procedures become less morbid with decreased operative times, less postoperative pain, and quicker recovery, the benefits of hospital admission become less significant and same-day discharge is possible without compromising outcomes, patient safety, or satisfaction.8–10 Outpatient procedures inherently cost less and result in significant savings and decreased utilization of hospital beds.11 Surgeries can be moved to ambulatory surgery centers (ASCs), which have documented benefits with regard to efficiency, cost, patient and surgeon satisfaction, scheduling flexibility, and possibly reduced complications compared with the hospital setting (HS).12–14 These are critical factors in a healthcare system which is becoming increasingly less efficient and cost-effective.15–17
The most feared complication after implant-based postmastectomy reconstruction is infection and reconstructive failure, increasing patient distress and healthcare costs.18,19 Infections result in prolonged hospital admissions and repeated surgical procedures with inferior aesthetic outcomes. Khansa et al20 published a standardized best-practices protocol to reduce infections after tissue expander (TE) reconstruction. Despite following this protocol and implementing recommendations from several other recent reports,21–23 our reconstructive failure rate secondary to infection was over 10% in the HS. We felt that there were likely variables out of our control in the HS that might be contributing to our increased incidence of infections and that changing venue might reduce infectious complications.
Although several reports in other surgical fields24–27 have compared outcomes between ASCs and the HS, we are only aware of one similar publication by Oxley et al28 in the field of immediate implant-based postmastectomy reconstruction, which demonstrated equivalent rates of complications and outcomes between the 2 venues. The feasibility of routinely performing mastectomy and immediate implant-based reconstruction with same-day discharge using an enhanced recovery protocol was established by Dumestre et al10 in 2017. The present study was designed to evaluate outcomes, complications, and patient satisfaction of immediate prepectoral TE reconstruction performed in an ASC with same-day discharge compared with the identical procedure performed in an HS with or without hospital admission.
METHODS
Study Population
We performed a retrospective review of all consecutive mastectomy and immediate prepectoral TE reconstructions by a single surgeon between August 2014 and September 2019. This study was approved by the Gwinnett Surgical ASC/Northside Hospital Institutional Review Board (Lawrenceville, Ga.). Patients with subpectoral reconstructions, a history of radiotherapy, active smokers, and diabetics with Hemoglobin A1C >7.0 were excluded. Active smokers were required to stop smoking for 1 month before surgery. All surgeries had a minimum of 6-month follow-up. Surgery performed between August 2014 and June 2017 was exclusively performed in the HS with planned overnight observation. Between July 2017 and September 2019, surgeries were performed in either ASC or HS. Surgery was performed in the hospital if required for insurance reimbursement or requested by the patient. We performed the surgical procedures in an identical fashion regardless of whether we used the ASC or HS.
Mastectomy and Reconstructive Surgery
We followed the protocol outlined by Khansa et al20 and others21–23 for patients undergoing TE reconstruction after mastectomy to minimize infectious complications. All reconstructions used any one of the 3 different thick acellular dermal matrices (ADMs): AlloDerm (reference 102320; LifeCell/Allergan, Bridgewater, N.J.), FlexHD (reference HP1620; MTF Biologics, Edison, N.J.), and Cortiva (reference DH 1620; RTI Surgical, Alachua, Fla.) motivated by cost and availability. TEs used were the Allergan 133 series (reference 133 SX or 133 MX; Allergan Plc., Bridgewater, N.J.) until 2019 when a transition to smooth Mentor CPX4 TEs (reference 350-9211-9216; Johnson & Johnson, Santa Brarbara, Calif.) was introduced to reduce infection rates.
The mastectomy surgery was performed through an inframammary (IMF) incision unless the nipple was being excised or there were oncologic concerns regarding a skin margin. Less commonly, skin-reducing, Wise-pattern mastectomy surgeries were performed as well. After the mastectomy surgery was completed, a 16 × 20 cm2 ADM was perforated and then washed in saline and then soaked again. The ADM was sutured to the prepectoral pocket along the IMF and lateral breast border, using 2-0 Vicryl (reference JB259; Ethicon, Inc., Somerville, N.J.). The pocket was then washed with a cefazolin, gentamicin, and bacitracin solution followed by several rinses with a 50% betadine solution. The skin was then prepared with a povidone-iodine solution and redraped before TE placement. The expander was immersed in the triple-antibiotic solution, and all gowned operating room personnel changed gloves. The expander was inserted into the mastectomy pocket minimizing skin contact and sutured to the chest wall, using 2-0 Vicryl (reference JB259; Ethicon, Inc., Somerville, N.J.). The ADM was then draped over the anterior surface of the TE securing it with sutures to the superior and medial borders of the breast footprint. The expanders were filled with air or saline, minimizing tension on the mastectomy flap. Two closed-suction 15-round Blake drains were placed and finally removed after drain output was <30 mL over 24 hours. Patients were given a dose of preoperative cefazolin, which was continued for 24 hours in the HS. Patients continued with cephalexin until all drains were removed. Patients were discharged after 24-hour observation in the HS and after 2 hours of monitoring in the ASC.
Data Collection
Charts were reviewed to obtain demographic data and comorbidities, body mass index (BMI), history of tobacco abuse, intent of surgery (curative versus prophylactic), location of surgery (ASC or HS), the extent of axillary surgery, type of mastectomy (skin- versus nipple-sparing), operative time, unilateral versus bilateral surgery, the use of textured versus smooth TEs, mastectomy weight, neoadjuvant or adjuvant chemotherapy, adjuvant radiotherapy, and length of hospital stay. We also collected data on patient satisfaction with overall surgical experience using a 5-point Likert scale assessing the results of the following 3 questions (1 = strongly disagree, 2 = disagree, 3 = undecided, 4 = agree, 5 = strongly agree):
We then categorized our results into 2 patient groups: those patients who underwent surgery at our ASC with same-day discharge and those who had their surgery performed in the HS with or without hospital admission. The primary outcomes of interest were complications, reoperation for any reason within 180 days (other than for implant exchange), and reconstructive failure. We defined major complications as the following: seroma or hematoma that required operative drainage, skin flap necrosis as any area of full-thickness necrosis that required operative debridement or delayed expansion, nipple necrosis that required surgical debridement or delayed expansion, dehiscence as any wound separation of >5 mm unrelated to necrosis or infection that required reoperation, infections as those that required hospital admission for intravenous antibiotics or operative intervention for removal or salvage of the expander. Reconstructive failure was defined as removal of the TE with or without replacement. Reoperations included those for debridement of mastectomy flap necrosis, evacuation of hematomas or seromas with drain placement, and washout and replacement or removal of a TE. Minor complications were those could be managed in the office setting.
Statistical Analysis
All data were collected and reviewed by the author. Statistical analysis was performed using IBM SPSS Version 23.0 (IBM Corp., Armonk, N.Y.). Univariate analysis was used to compare HS and ASC patients. Frequencies and proportions were calculated for categorical variables, and comparisons were made with χ2 analysis or 2-tailed Fisher exact test. Means and SDs were calculated for continuous variables, and comparisons were made using independent sample t tests. All comparisons were unpaired. All percentages were calculated based on the total number of reconstructed breasts. Type I errors of <5% (P < 0.05) were used to determine statistical significance, and all reported P values were 2-tailed comparisons. Univariate analysis was used to determine risk factors of significance and confounding variables to build a model for multivariate logistic regression analysis. Multivariate logistic regression analysis was performed to determine the impact of location of surgery on infectious complications leading to implant salvage or removal. A P value of <0.05 was considered significant.
RESULTS
Over 62 months, 209 women underwent mastectomy and prepectoral TE reconstruction. This included 103 patients (177 breasts) at an ASC with same-day discharge and 106 patients (183 breasts) in the HS who were usually admitted (94 of 106 patients) for observation. Data were compared between the 2 groups (Table 1).
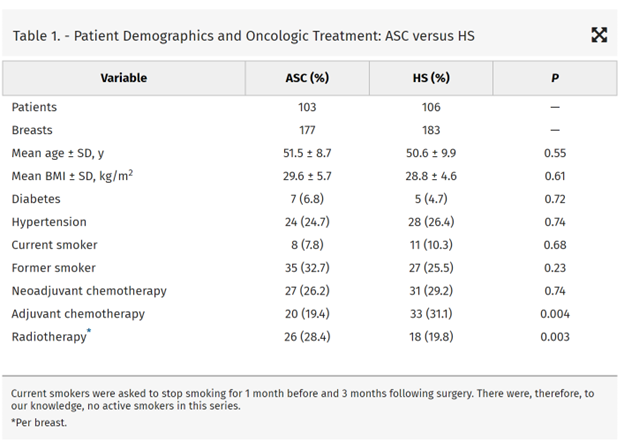
There were no significant differences between the HS or ASC groups in mean age or BMI, or the proportion of patients who were smokers, diabetics, or had a diagnosis of hypertension. Although the percentage of patients undergoing neoadjuvant chemotherapy was similar between the 2 groups, the percentage of patients undergoing adjuvant chemotherapy was higher in the HS [31.1% (n = 33) versus 19.4% (n = 20); P = 0.004]. The percentage of breasts undergoing adjuvant radiotherapy was higher in the ASC [28.4% (n = 26) versus 19.8% (n = 18); P = 0.003]. The percentage of patients undergoing bilateral versus unilateral surgery and skin-sparing versus nipple-sparing versus skin-reducing mastectomy was not significantly different between the 2 locations (Table 2). The extent of axillary surgery was also similar between the 2 venues as well. Operative times were significantly shorter in the ASC (138.6 ± 22.7 versus 160.2 ± 31.2 minutes; P = 0.02). The mastectomy specimen weights were similar between the 2 locations as were the rates of therapeutic versus prophylactic mastectomy. Textured TEs were also more commonly used in the HS compared with the ASC [89.1% (n = 163) versus 61.4% (n = 121); P = 0.00001].
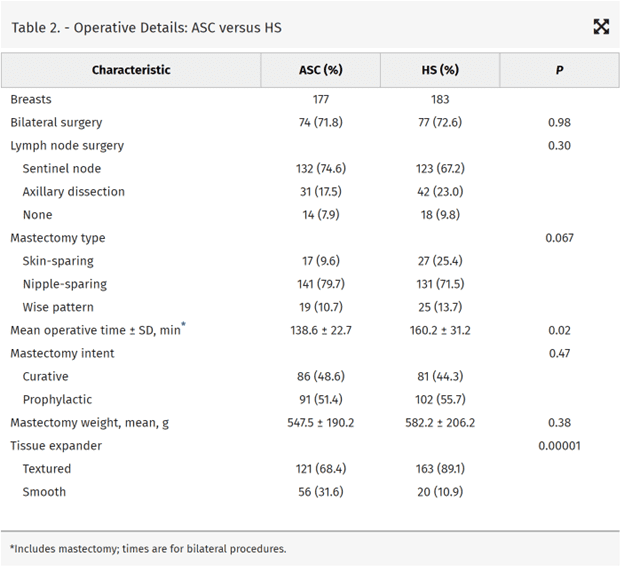
The average follow-up time was 28.7 ± 6.4 months in the ASC and 36.2 ± 9.1 months in the HS (P = 0.02) with shorter mean hospital length of stay (0.01 ± 0.10 versus 0.88 ± 0.45 days; p < 0.000001) (Table 3). Twelve of 106 patients were discharged from the HS on the day of surgery. Although the rate of minor complications was similar between the groups, there were significantly fewer major complications in the ASC [20.2% (n = 37) versus 10.7% (n = 19); P = 0.03]. The rates of major complications, including seroma, hematoma, and skin flap necrosis (includes nipple necrosis) requiring reoperation or intervention, were similar. Major infectious complications were more prevalent in the HS compared with the ASC [n = 21 (11.5%) versus n = 4 (2.3%); P = 0.001], which included more frequent surgical intervention for implant removal or salvage. There was no differences between the groups with regard to aborted reconstruction and the rates of successful implant-based reconstruction. One patient who had surgery in the HS required hematoma evacuation which was realized before discharge. We had 2 of 103 patients (1.9%) at the ASC who required unplanned readmission for hematoma evacuation. There were no unplanned readmissions from either location for control of postoperative pain or nausea.
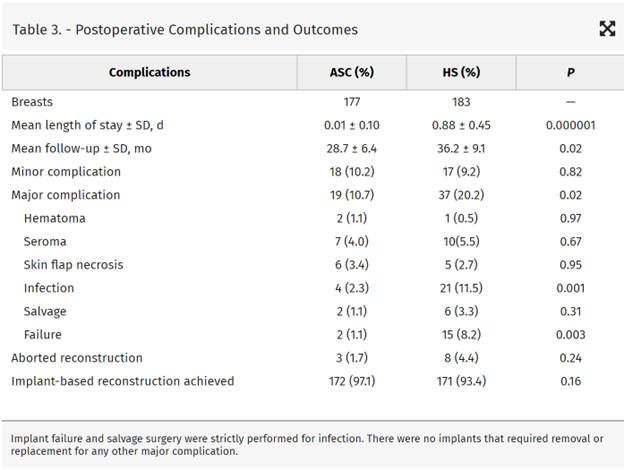
Univariate analysis demonstrated that BMI, diabetes, smoking history, extent of axillary surgery, adjuvant radiotherapy, neoadjuvant chemotherapy, and surgery location predicted major infectious complications requiring surgical intervention (Table 4).
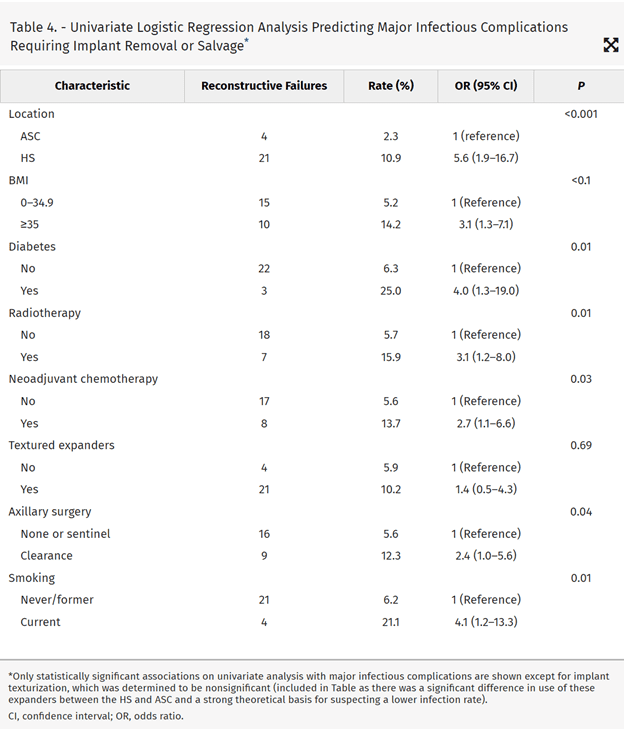
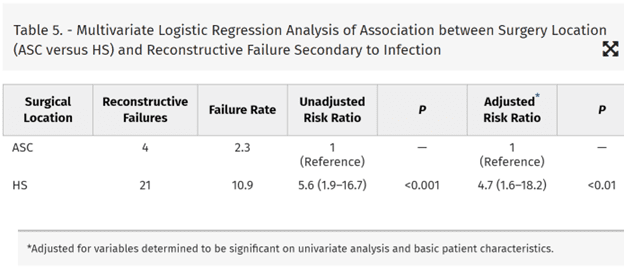
Smooth surface TEs did not have a reduced infection rate. Multivariate analysis (controlling for variables determined significant on univariate analysis and basic patient demographics and the use of adjuvant chemotherapy) revealed that the HS predicted a rate of infectious complications requiring surgery that was 4.7 times higher than in the ASC (odds ratio, 4.7; 95% confidence interval, 1.6–18.2) (Table 5).
Finally, we assessed patient satisfaction using a 5-point Likert scale with overall surgical experience and found that patients in the ASC were equally comfortable and prepared for discharge after surgery compared with patients having surgery in the HS (4.42 ± 0.42 versus 4.58 ± 0.36; P = 0.62). Patients having surgery in the ASC were both more likely to recommend their surgical experience to others (4.81 ± 0.51 versus 4.11 ± 0.32; P = 0.11) and have their surgery again in the same location (4.72 ± 0.42 versus 3.99 ± 0.51; P = 0.02) if given the option of changing operative venue.
DISCUSSION
Recent advances in surgical oncology (preservation of the nipple and subcutis) and reconstructive breast surgery (ADM, prepectoral reconstruction, lipofilling, intraoperative vascular assessment, cohesive implants) have improved outcomes and decreased the morbidity of implant-based reconstruction,1–5,29–31 often allowing for same-day discharge after surgery. Despite these advances, infections, resulting in reconstructive failure, have a documented rate between 2.5% and 24%.18,19,21–23,32 Khansa et al20 and others21–23 have published standardized best-practice protocols for preoperative, intraoperative, and postoperative measures to reduce infections. Despite following these protocols, we had a reconstructive failure rate secondary to infection of 10%. We reasoned that there were likely causes of infection that were beyond our control in the HS, including the adequacy of the sterilization processes, the experience, specialization, and knowledge of the variable personnel provided us; the prevalence of drug-resistant organisms; and the routine performance of contaminated cases that resulted in increased infections. The HS also involves significant additional traffic through the operating room as new scrub technicians, circulating nurses, surgical first assistants, and anesthesia personnel change shift, possibly contributing to an increased infection risk. We felt that these factors were all favorably addressed in our ASC (shift changes are not allowed) and that changing venue might lead to reduced infections.12–14
With the advent of prepectoral breast reconstruction, we felt that patients could be discharged as they had reduced postoperative pain. We began to offer patients outpatient mastectomy and reconstruction in the ASC in July 2017 and realized our infectious complications were reduced. One year later, we presented this as the default option to all patients who desired implant-based reconstruction. Eighty percent of our implant-based reconstructions are now performed in the ASC, with the remaining 20% performed in the HS because of insurance coverage issues (15%) or patient request (5%). When patients are presented with an infection rate one fifth that of the hospital and a record of performing these procedures with good outcomes, few have qualms about proceeding with surgery and same-day discharge. This is a critical component of the pathway in setting patients’ expectations that same-day discharge is the norm and not the exception.28 When reassured, patient fears regarding infectious complications far supersede concerns over postoperative pain and nausea. Patients receive a surgical packet, including preoperative and postoperative instructions, drain care, pain management, prescriptions, and on-call provider contact numbers.10
This report has obvious relevance to those surgeons who, despite using best-practice guidelines, still have a high reconstructive failure rates secondary to infection. Surgeons are held responsible for their infection rates but are not in full control of the processes and variables in the HS that impact these rates. These risk factors may persist despite meticulous attention to sterility by the surgeon. Shifting the surgery to an ASC gives the surgeon more control of the process. Different HS may be more geared toward breast reconstruction, and some surgeons may not witness the same reduction in infectious complications if their rates are already low. These surgeons may still help preserve precious hospital beds for more acutely ill patients by discharging their patients after surgery. This report is also timely given the recent coronavirus disease 2019 pandemic. Shifting surgery to an ASC is safer for immunocompromised patients who have or will be receiving chemotherapy. Decreasing healthcare costs will be crucial, and this report demonstrates no benefit to hospital admission after surgery. As ASCs are inherently more cost-effective and with a possibly reduced infection rate as demonstrated here, shifting surgery to the ASC will have the most pronounced impact on reducing costs and decreasing utilization of resources.
Our patients feel comfortable and prepared for same-day discharge from the ASC. They are satisfied with their experience, and the majority would repeat the process and avoid the HS. Most would recommend surgery in the ASC to others over the HS. Our rates of immediate postoperative complications requiring reoperation are very low, arguing against observation for this reason. We have had no readmissions for pain control or nausea.
Reduced infection rates in ASCs have been reported in the orthopedic literature.12–14 This is the first report to demonstrate that immediate, implant-based postmastectomy reconstruction has a reduced rate of infection in an ASC versus HS. The single previous publication comparing immediate implant-based reconstruction in an ASC versus HS demonstrated no differences in complications.28 However, this was a hospital-owned ASC and may have been run with similar protocols and procedures in place as the main hospital which might minimize differences in outcomes. Their infectious complication rates may have already been low, and a difference may have been difficult to detect between the 2 venues. The details of the surgeries performed were not reported with regard to nipple- versus skin-sparing mastectomy. In their series, unilateral and bilateral mastectomy and reconstruction took 68 and 78 minutes, respectively. Our surgical times were nearly twice as long and a recent report from an established plastic surgery department in the United States documented operative times that were 4 times as long.33 The majority of our mastectomies were nipple-sparing procedures performed through IMF incisions (Figs. 1–6). Given the abbreviated operative times in the previous report, these were unlikely nipple-sparing procedures and a direct comparison is difficult.
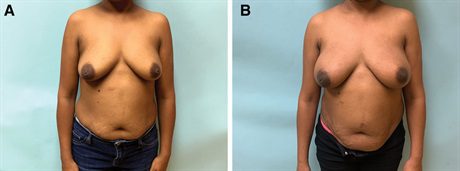 Fig. 1.:
Fig. 1.:
A fifty-five–year-old woman with left breast cancer. A, She undergoes a bilateral mastectomy and prepectoral reconstruction through inframammary fold incisions. A, Before photograph. Six months later, she undergoes exchange for smooth, round, high-projection silicone implants with fat transfer. B, Photograph taken 6 months after the implant exchange.
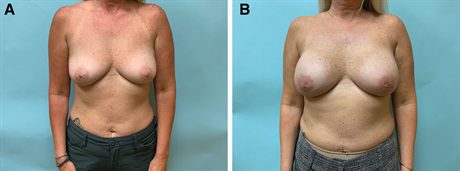 Fig. 2.:
Fig. 2.:
Before and after photographs of a forty-nine–year-old woman who underwent bilateral prophylactic mastectomy and reconstruction. A, Before the exhange, she desires an increase in breast size. B, Her final postoperative photograph is taken 10 months after exchange of her tissue expanders for smooth, round, high-projection implants and fat transfer.
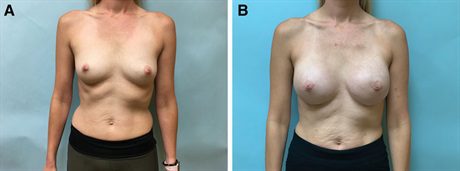 Fig. 3.:
Fig. 3.:
-nine–year-old woman with bilateral breast cancer desired mastectomy and an increase in breast size. A, Before photograph. B, Her photograph is taken 4 months after her final surgery, which involves exchange of her tissue expanders for definitive implants and fat transfer.
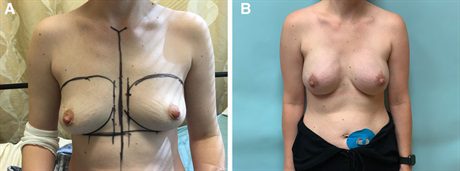 Fig. 4.:
Fig. 4.:
A thirty-seven–year-old woman with left breast cancer desires a “lifted appearance” with a modest increase in breast size. A, Before photograph. B, Her photograph is taken 5 months after exchange of her tissue expanders for anatomic, low-height, extra-projecting silicone implants with fat transfer.
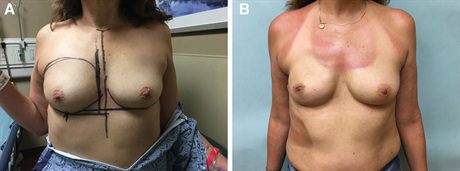 Fig. 5.:
Fig. 5.:
A forty-two–year-old woman with right breast cancer underwent a unilateral mastectomy and immediate prepectoral reconstruction. She refused a contralateral symmetry surgery. A, Before photograph. B, Her photograph taken 6 months postoperative after fat transfer and placement of an anatomic, low-height, moderate projection silicone implant. Her result would be improved with a wider implant, but she refused revision.
 Fig. 6.:
Fig. 6.:
A seventy-two–year-old woman with left nipple inversion and Paget’s disease undergoes a skin-sparing mastectomy and immediate prepectoral reconstruction. A, Before photograph. B, In the postoperative photograph, she is shown 2 months after a right mastopexy for symmetry and exchange of her left tissue expander for a definitive implant.
The limitations of this study include its retrospective nature and single institution design with a single surgeon which increases potential for surgical bias. Although technical variables were controlled by the fact that a single surgeon performed all the surgeries in this study, it is possible that small improvements in technique over time may have led to reduced infections in the ASC that may not be attributed to changing surgical venue alone. This is an especially relevant consideration here, as the author only began performing his own mastectomy and reconstructions at the beginning of the study period. Additional studies in different HS and ASCs (including multiple breast surgical oncologists and reconstructive surgeons with larger sample sizes) are required to confirm our findings.
CONCLUSIONS
Mastectomy and implant-based prepectoral reconstruction with same-day discharge is feasible with low complication and readmission rates and excellent patient satisfaction. This allows for use of an ASC, which has resulted in a significant reduction in infectious complications and reconstructive failure in our experience. These results have important implications for cost containment and reducing exposure of breast cancer patients to nosocomial infections in the hospital. Same-day discharge frees up beds in the hospital for the more acutely ill. We maintain that hospital admission after mastectomy and immediate prepectoral reconstruction should be the exception and not the rule.
REFERENCES
Jean-Claude Schwartz, MD, PhD – Breast Cancer and Reconstructive Surgeon

As a national leader in oncoplastic breast surgery, Dr. Schwartz has developed and published some of the most innovative ways to save women’s breasts, allowing them to avoid mastectomy and multiple reconstructive surgeries. Dr. Schwartz’s techniques have benefitted his patients and other surgeons around the world.
There is no breast surgical oncologist in the United States that offers the same variety of oncoplastic options, nor has contributed more to the different fields of breast reconstruction than Dr. Schwartz.