Fax: 770-339-9804
Lawrenceville, Georgia 30046

Originally published on PRS Global Open website in November 2022
Abstract
Background:
Nipple-sparing mastectomy (NSM) and direct-to-implant reconstruction (DTIR) allow patients to complete their surgical care in one surgery. However, for women with significant ptosis, NSM is frequently not offered or requires multiple procedures.
Methods:
We performed a retrospective review of a single-surgeon practice from 2016 to 2021 of a single-stage, modified, bidirectional adipodermal mastopexy to facilitate NSM and DTIR in patients with breast cancer and grades 2-3 ptosis. Demographics, intraoperative details, and postoperative outcomes were recorded. We also conducted a literature review and compared our technique to previously published approaches.
Results:
Sixty breast cancer patients (105 breasts) with grades 2-3 ptosis underwent NSM and prepectoral DTIR using this technique. The average nipple-areola complex (NAC) lift was 9cm (range, 4 -15cm), and the average preoperative nipple to inframammary fold distance was 12cm (range, 8 -17cm). Overall complications included seroma [n = 8 (8%)], T-junction dehiscence [n = 6 (6%)], mastectomy flap necrosis [n = 6 (6%)], and superficial/partial NAC necrosis [n = 2 (2%)] with no incidence of complete NAC necrosis. Comprehensive literature review confirmed that the modified, bidirectional adipodermal mastopexy has a favorable complication profile when compared with other previously described approaches despite its application to more challenging patient populations undergoing DTIR.
Conclusions:
The modified bidirectional adipodermal mastopexy safely facilitates NSM and DTIR in breast cancer patients with ptosis without requiring multiple procedures or leaving behind breast tissue and, in our hands, is the preferred approach in this difficult patient population.
Takeaways
Question: How can we safely preserve the nipple areola complex in patients with significant ptosis and macromastia?
Findings: The modified bidirectional adipodermal mastopexy described here, a modification of the McKissock bipedicle breast reduction technique, allows us to safely preserve the nipple in patients with significant ptosis and macromastia undergoing mastectomy, with improved outcomes compared to previously described techniques.
Meaning: Breast cancer patients with significant ptosis and macromastia are candidates for nipple-sparing mastectomy and direct-to-implant reconstruction using the modified bidirectional adipodermal mastopexy approach without significant rates of complication.
INTRODUCTION
Nipple-sparing mastectomy (NSM) has revolutionized aesthetic outcomes after breast cancer surgery.1 The added innovations of prepectoral reconstruction, acellular dermal matrix (ADM), and lipotransfer have further improved outcomes after implant-based reconstruction.2 Many patients who have sufficient subcutaneous tissue after mastectomy will have results that rival a cosmetic breast augmentation.3
Outstanding results are best obtained in patients with grades 0, 1, or mild grade 2 ptosis, where skin retailoring and nipple repositioning are unnecessary.4 Breast cancer patients with significant ptosis are usually not candidates for NSM and direct-to-implant reconstruction (DTIR), as the mastectomy devascularizes the nipple and skin flaps with the additional insults of repositioning and insetting the NAC, incising and reducing the skin envelope, and the implant weight further increasing the risk of complications.
The optimal solution for these patients would be where a mastopexy and NSM could be performed with maintenance of vascularization of the NAC and Wise-pattern mastectomy flaps (WPMFs) without resorting to surgical delays, leaving behind subareolar tissue/thicker flaps, staged mastopexy surgery, or tissue expanders (TEs).5–10 Here, we utilize an approach based on the McKissock bipedicle11 used in mastopexy and breast reduction surgery to perform the skin-only mastopexy, NSM, and DTIR in breast cancer patients with grades 2 or 3 ptosis. By using a bidirectional adipodermal pedicle and keeping incisions to minimum lengths at strategic locations along the mastopexy pattern, we preserve blood flow and minimize the risk of tissue necrosis. Here, we describe 60 consecutive patients (105 breasts) using this approach and compare our outcomes to those previously described with comprehensive literature review of this topic.
METHODS
Retrospective review of a single surgeon (J.S.) practice performing both the NSM and reconstruction from 2016 to 2021 in one ambulatory surgery center with at least 6 months follow-up was performed. All patients had a breast cancer diagnosis and grades 2-3 ptosis and underwent unilateral or bilateral NSM, bidirectional adipodermal mastopexy, and prepectoral DTIR. Patients who underwent unilateral NSM underwent simultaneous symmetry surgery. Delayed reconstructions, prophylactic surgery, autologous reconstruction, or other incision types were excluded. Smokers, patients with poorly controlled diabetes, and patients with a history of radiotherapy were not offered this approach. Demographics, procedural details, additional adjuvant treatments, and complications were recorded. Minor complications were those that were addressed in the ambulatory setting, whereas major complications required either hospital admission or general anesthesia.
We conducted a PubMed review using the key words and phrases NSM and ptosis, NSM and mastopexy, NSM and macromastia, skin-reducing mastectomy, and reconstruction. We excluded studies that included autologous reconstructions or required a surgical delay. We excluded studies performed before 2000 when NSMs were “subcutaneous,” leaving tissue behind to preserve blood flow. We excluded periareolar mastopexies, which limit skin retailoring and nipple repositioning. We included publications reporting on at least five breasts, where the mastectomy, nipple repositioning, skin retailoring, and reconstruction were performed in one surgery. We further reviewed the references of each article from this search to find additional relevant articles. We performed a similar search on the websites of Plastic and Reconstructive Surgery, Plastic and Reconstructive Surgery Global Open, Annals of Plastic Surgery, and Journal of Plastic Reconstructive and Aesthetic Surgery. All articles were then analyzed for the following: number of patients and breasts, subpectoral versus prepectoral reconstruction, TE versus DTIR, nipple necrosis rate, mastectomy flap necrosis rate, average mastectomy weight, average implant size, average sternal notch to nipple distance, average nipple to inframammary fold (IMF) distance, and type of mastopexy pattern used. We then compared our outcomes using the modified bidirectional adipodermal mastopexy to those previously reported in the literature.
Surgical Technique
Patients are marked with a Wise-pattern, placing the NAC at the level of the IMF. All skin within the Wise-pattern is deepithelialized, sparing the NAC. All deepithelialized tissues are preserved, along the vertical limbs, around the NAC, and at the IMF, which both optimizes blood flow and reinforces the suture line. The mastectomy is performed through two incisions—the medial and lateral extensions to the IMF from the distal ends of the medial and lateral vertical limbs, respectively (Fig. 1). All breast tissues are removed by following the superficial fascial system, preserving the subcutaneous tissue and blood supply. The NAC is elevated just beneath the level of the deep dermis, and no retroareolar breast tissue is intentionally preserved. A retroareolar tissue specimen is sent for frozen section, and the NAC is removed if positive for carcinoma but preserved if negative or equivocal. It is important that the breast surgeon regularly alternates between both sides of the operating room table to optimize visualization and exposure, reducing the difficulty of the dissection. In addition, to improve visualization, the incision from the lateral vertical limb to the IMF can be extended even further laterally for better exposure. The bidirectional adipodermal flap should never be pulled on aggressively. Instead, when tension must be applied, both incisions from the medial and lateral vertical limbs to the IMF, through which the dissection is performed, can be judiciously retracted without risk of compromising the blood supply to the NAC.
 Fig. 1.:
Fig. 1.:
Intraoperative technical details. Here, we see a representative patient after the mastectomy has been completed (A) through both the medial (black arrow) and lateral (yellow arrow) extensions to the inframammary fold from the distal ends of the medial and lateral vertical limbs, respectively. A prepectoral implant sizer is placed into position. The bipedicle flap is intact with excellent bleeding. Almost all suture lines are reinforced by viable tissue, and the nipple is not located at the distal end of a flap, increasing its vascularity. As opposed to previous bipedicle descriptions, the tissue along the medial and lateral vertical limbs is never incised unless this is necessary for NAC mobility—but we never need to incise more than one-third of the vertical limb. B, We see that working through these limited incisions allows us to create two spaces, labeled 1 and 2, after the mastectomy is completed. Space 2 is the lateral space through which lymph node dissection and implant placement are performed.
The medial and lateral vertical limbs are never incised during the mastectomy to preserve blood flow to both the NAC and WPMF. This creates a bidirectional adipodermal flap, providing significant implant coverage from the deepithelialized and involuted tissue. After the mastectomy, the NAC and WPMFs are evaluated by the Kent system (Kent Imaging, Calgary, Alberta, Canada), which assesses tissue oxygenation. The reconstruction is aborted if tissue oxygenation is unacceptable. If tissue oxygenation is acceptable, the lateral border of the breast is reconstructed with ADM. A prepectoral implant sizer is placed, the incisions closed, the NAC inset and tissue oxygenation assessed, and if questionable, the NAC is harvested as a free nipple graft or a deflated TE can be attempted. Well-perfused NACs are kept on the bidirectional flap. Often‚ the dermis along both the distal medial and lateral vertical limbs (Fig. 1) is partially incised to allow for easier movement of the NAC as the bidirectional flap makes this more challenging than unidirectional approaches. This is done in a stepwise fashion to find just the required amount of NAC mobility while simultaneously minimizing devascularization. An ADM (16 cm × 20 cm)-implant construct is assembled ex vivo with spanning sutures across the posterior surface of the implant. All reconstructions used any one of the three different ADMs: AlloDerm (reference 102320; LifeCell/Allergan, Bridgewater, N.J.), FlexHD (reference HP1620; MTF Biologics, Edison, N.J.), and Cortiva (reference DH 1620; RTI Surgical, Alachua, Fla.) motivated by cost and availability. Then, based on the width of the implant, the lateral border of the reconstructed breast is marked and reinforced by suturing the ADM-implant construct down to the serratus to establish the lateral breast fold. No other border is secured as the IMF, and medial breast border is meticulously preserved. Posterolateral tissue is also advanced anteriorly and medially and secured to the chest wall with absorbable suture to further aid in reducing the risk of lateral implant malposition and postoperative seroma.
A single 15-round Blake drain is inserted laterally. Drains are discontinued when output is less than 30 ml over 24 hours. Cephalexin is prescribed postoperatively and continued for 7 days. Five representative cases are presented in Figures 2–6.
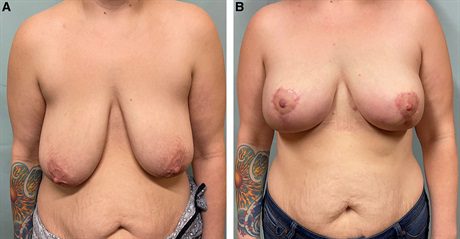 Fig. 2.:
Fig. 2.:
A 44-year-old woman with multicentric left breast cancer, breast asymmetry, and grade 2 ptosis undergoes bilateral mastectomy, bipedicle mastopexy, and prepectoral direct-to-implant reconstruction. A, Preoperative photograph. B, She is shown 4 months postoperatively.
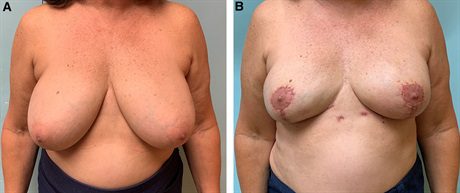 Fig. 3.:
Fig. 3.:
A 60-year-old woman with bilateral breast cancer and grade 2 ptosis undergoes bilateral mastectomy, bipedicle mastopexy, and prepectoral direct-to-implant reconstruction. A, Preoperative photograph. B, She is shown 3 months postoperatively.
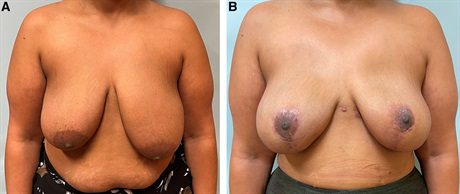 Fig. 4.:
Fig. 4.:
A 43-year-old woman with left breast cancer, grade 3 ptosis, and asymmetry undergoes bilateral mastectomy, bipedicle mastopexy, and prepectoral direct-to-implant reconstruction. A, Preoperative photograph. B, She is shown 8 months postoperatively.
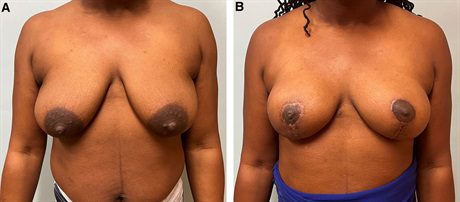 Fig. 5.:
Fig. 5.:
A 45-year-old woman with multicentric right breast cancer and grade 2 ptosis undergoes right mastectomy, bipedicle mastopexy, and prepectoral direct-to-implant reconstruction with simultaneous left breast McKissock bipedicle reduction. A, Preoperative photograph. B, Her symmetry is excellent because we have used the same incision and pedicle for both the mastectomy and reduction. The use of a less form stable implant placed into the prepectoral plane also optimizes symmetry as we have shown previously.
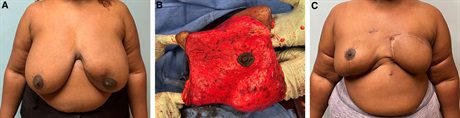 Fig. 6.:
Fig. 6.:
Nipple-sparing mastectomy in a patient with significant macromastia and ptosis. A, A 44-year-old morbidly obese woman with locally advanced left breast cancer with a poor response to chemotherapy. She requires excision of most of her left breast skin to clear her cancer, which is replaced with a muscle-sparing latissimus flap. She desires right nipple preservation. B, We use the bipedicle mastopexy technique. The black arrow represents the distal medial vertical limb, the yellow arrow represents the distal lateral vertical limb, and the blue arrow represents the new NAC position. The NAC is centrally located within a large expanse of well vascularized tissue and not located at the end of a flap, which would compromise its vascularity. The vertical limbs do not require incision for NAC mobility as there is enough redundancy in the tissues for significant mobility. C‚ We see her 6 months after her right NSM, bipedicle mastopexy and DTIR. She is awaiting left NAC reconstruction.
RESULTS
Demographics, oncological characteristics, and anatomic features of the patient cohort are presented in Table 1.
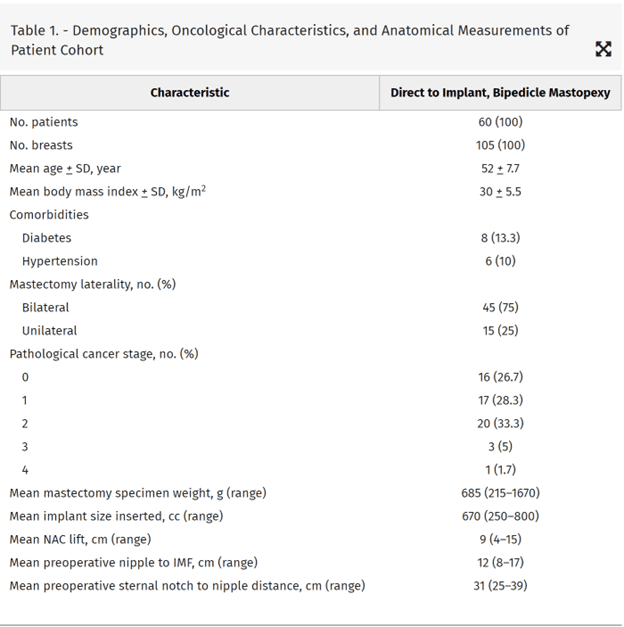
The mean patient age, body mass index, and follow-up was 52 years (range, 26–75 years), 29.6 kg/m2 (range, 20–41 kg/m2), and 12 months (range, 6–57 months), respectively. The average NAC lift was 9 cm (range, 4–15 cm), preoperative nipple to IMF distance was 12 cm (range, 8–17 cm), and preoperative sternal notch to nipple distance was 31 cm (range, 25–39 cm). Breast cancer staging was recorded as stage 0 (16), stage 1 (17), stage 2 (20), stage 3 (6), and stage 4 (1). Neoadjuvant chemotherapy, adjuvant chemotherapy, and postmastectomy radiotherapy were delivered to 18%, 23%, and 17% of patients, respectively. No patients required a free nipple graft as all had satisfactory tissue oxygenation assessment. The average mastectomy weight and implant volume was 685 grams (range, 215–1670 grams) and 670 cc (range, 250–800 cc), respectively.
Complications are documented in Table 2. Two (2%) breasts developed partial-thickness NAC necrosis and healed by secondary intention, and eight (8%) developed seroma, two of which required operative drainage. Six (6%) breasts developed T-junction dehiscence, two of which underwent immediate successful surgical revision and the remainder of which healed with wound care. Six (6%) breasts developed mastectomy flap necrosis, two of which required latissimus dorsi salvage and the remainder of which healed with outpatient wound care. Two (2%) breasts developed periprosthetic infections (unrelated to wound healing complications) and underwent implant salvage with washout, removal, and prosthetic replacement.
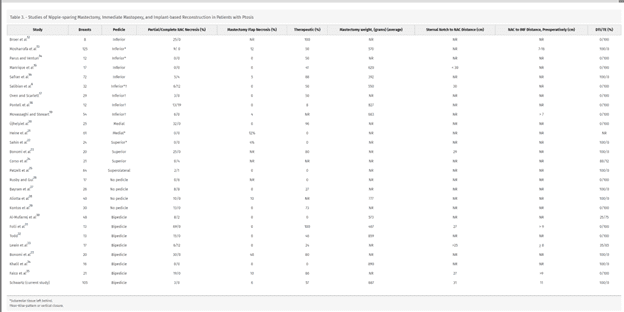
The literature review identified 25 articles (Table 3), which fell into several reconstructive patterns. Most studies described a discrete, unidirectional, or bidirectional adipodermal flap through which the NAC was supported and repositioned6,12–31 and used a Wise or vertical mastopexy pattern for skin retailoring. Other studies did not commit to a unidirectional adipodermal pedicle and kept most attachments intact,32–35 which limited the extent of nipple repositioning possible. Some studies modified the mastopexy pattern to eliminate the T-junction and placed an incision in the IMF6,17 or across the breast18,19,28,35 to reduce the incidence of mastectomy flap/T-junction necrosis. The adipodermal flaps described were inferiorly based (9)‚6,12–19 bidirectional (8),23,27–31 superiorly based (4),22–25 medially based (2),20,21 and no direction specified (4).32–35 The inferiorly based adipodermal pedicle reports included four descriptions that were not Wise-patterns,6,17–19 eliminating the vertical scar and placing the scar across the center of the breast18,19 or at the IMF.6,17 Three inferiorly based adipodermal pedicle reports describing leaving subareolar tissue behind6,13,14 to support NAC perfusion, one of which required a second surgery for removal.6 Although the descriptions of the superiorly22,25 and medially based adipodermal pedicles21 also included reports that described leaving behind subareolar tissue, none of the described bidirectional approaches included this modification. Direct comparison of these different techniques is difficult given that the approaches are applied to different patient populations (therapeutic versus prophylactic surgery), may require one (DTIR) or two (TE) procedures, and may or may not leave behind subareolar tissue (Table 2). In addition, these approaches are applied to patients with different degrees of ptosis or macromastia (in many cases, these variables are not reported), which makes comparison of outcomes challenging. Regardless, the modified, bidirectional adipodermal pedicle described here applied to breast cancer patients with significant ptosis and macromastia, performed in a single stage, in the prepectoral plane, has a lower rate of NAC and WPMF necrosis than other published studies despite the fact that many of these previous techniques were applied to less challenging patient populations (Table 2).
DISCUSSION
Significant ptosis has traditionally been thought to be a contraindication to NSM.4 To address this, Spear et al5 recommended a first-stage mammoplasty, where the cancer is removed with simultaneous NAC repositioning and retailoring of the skin envelope. Three months later, after the nipple has developed significant collateralization and the WPMFs have been delayed, NSM and TE or DTIR is performed. There are two drawbacks to this approach—the requirement for multiple procedures and the inability to use this for multicentric cancers. Patients with multicentric cancers require a mastectomy and cannot undergo first-stage mammaplasty/breast conservation surgery to reposition the nipple and retailor the skin envelope.
There lacks consensus on the best strategy to perform NSM and immediate mastopexy with several different techniques described. We reviewed all published reports on this topic to come to some conclusion as to what the best strategy might be. Using the methodology described in the Methods section, we identified 25 original publications6,12–35 that described an approach to NSM and simultaneous mastopexy in patients with ptosis. We discuss our outcomes compared with previous reports describing therapeutic NSM and immediate mastopexy, focusing on publications with the largest case numbers (Table 3).
Saliban et al6 and Oven and Scarlett17 reported on a mastopexy approach to facilitate NSM and TE reconstruction in ptotic patients based on the Passot or horizontal breast reduction,36 to avoid T-junction complications and a vertical scar. While their approach was applied to patients with significant ptosis and macromastia, they reported partial and complete nipple necrosis rates that ranged from 0% to 6% and 3.4% to 12%, respectively. We favor our approach with a lower rate of NAC necrosis, DTIR, and the use of Wise-pattern, which better retailors the skin envelope.
Mosharrafa et al13 described 125 Wise-pattern inferiorly based adipodermal pedicles with simultaneous NSM and DTIR in breast cancer patients. Their approach results in a 9% nipple necrosis rate despite leaving behind several millimeters of retroareolar breast tissue and the selective application of their technique to patients with less advanced cancers (no patients received neoadjuvant chemotherapy and one breast underwent postmastectomy radiotherapy). Our approach described here has a lower rate of nipple necrosis, does not leave behind subareolar breast tissue, and is applied to more advanced breast cancers.
Safran et al16 reported on 74 Wise-pattern inferior-based adipodermal pedicles, NSM, and DTIR. Five (5.1%) nipples had partial necrosis and four (4.0%) had complete necrosis. The mean implant size was 392 cc (range, 190–540 cc) (with no recording of mastectomy weights), suggesting the inclusion of mostly small to moderate-sized breasts. We favor our approach as it has a significantly lower NAC necrosis rate and is applied to larger breasted women.
Movassaghi and Stewart19 described the “smile mastopexy” in 54 patients undergoing therapeutic NSM and prepectoral TE reconstruction. Central breast skin was deepithelialized using a transverse “smile pattern” and then closure left a horizontal scar at Pitanguy’s point with the NAC supported on a broad-based inferior pedicle. Three (5.5%) nipples had partial necrosis, and there were two (3.6%) cases of mastectomy flap necrosis. Although their technique had a similar complication profile to our approach described here and was applied to a similarly challenging patient population, their approach is not ideal from an aesthetic standpoint as it leaves a transverse scar across the breast, and the extent of horizontal reduction is limited‚ resulting in wider reconstructed breasts. We acknowledge, however, that this approach may be more suitable for the highest risk patients (obese, diabetics, and smokers).
Aliotta et al34 reported on 40 breasts that underwent Wise-pattern (87.5%), J-pattern (7.5%) or vertical (5%) mastopexy, NSM, and DTIR. Two breasts (5%) had superficial nipple necrosis and two breasts (5%) had partial nipple necrosis. IMF incisions were made for access, and the Wise-pattern was then deepithelialized without additional incisions. This limited the amount of NAC elevation to less than 4 cm. This approach is limited to patients with mild ptosis with comparable rates of NAC necrosis to the approach described here.
Kontos et al29 reported on 30 breasts undergoing a batwing-style mastopexy, NSM, and TE reconstruction, leaving a transverse scar at the level of the repositioned NAC. There was no true directional flow to the adipodermal pedicle supporting the NAC as all the skin within the mastopexy pattern was deepithelialized and imbricated to accomplish the lift. Four (13.3%) breasts had partial nipple necrosis, three requiring operative debridement. Although this approach was applied to women with macromastia, breast cancer, and significant ptosis, it requires two stages, results in wider reconstructed breasts (no significant horizontal reduction is performed) with a transverse scar across the breast, and has a higher rate of NAC necrosis than our report here.
Our report described here is the largest published series of bipedicle adipodermal mastopexies to facilitate NSM and DTIR with the lowest documented rates of NAC necrosis. Our technique differs from other bidirectional adipodermal mastopexies in that full-thickness incisions are minimized through the medial and lateral vertical limbs. Previous reports described completely incising the medial and lateral vertical limbs with additional extension along both sides of the areola.23,26–31 Although this facilitates NAC mobility, it results in significant rates of NAC and WPMF necrosis as seen in previous publications. Tissue necrosis at the T junction and along the distal portions of WPMF is a known complication of Wise-pattern mastectomy surgery.37,38 Our approach preserves blood flow to the distal ends of the WPMFs as the adjoining segments of the mastectomy flap are left intact.
The bidirectional adipodermal mastopexy has a significant advantage over unidirectional approaches, as the NAC is not located at the distal end of the adipodermal flap where blood supply may be marginal. In prepectoral reconstructions, tissue necrosis in the vicinity of the NAC may lead to reconstructive failure as the prosthetic is located directly under the incision. The modified bidirectional mastopexy technique described here places the NAC in the center of a large extent of deepithelialized tissue (Fig. 6), even to a greater extent than previously described bidirectional approaches which fully incise the medial and lateral vertical limbs up to and past the areola.
We have attempted other skin-only mastopexy approaches, which have all resulted in unacceptably high rates of nipple necrosis (unpublished results). Others have had documented success with these pedicles, and we attribute this to intentionally leaving behind subareolar tissue/thicker mastectomy flaps, the use of TEs, applying these techniques to patients with less advanced cancers or prophylactic surgery, less significant ptosis, smaller breasts, and/or intentional preservation of the anterior intercostal artery in inferior pedicle approaches.16
CONCLUSIONS
We present here a modification of the Wise-pattern bidirectional adipodermal mastopexy that better preserves blood flow to the WPMFs and NAC. This results in improved outcomes compared with previously described bidirectional approaches without requiring TEs, leaving behind subareolar tissue, thick mastectomy flaps, or limiting its application to less advanced cancers or smaller breasts with less significant ptosis. The modified bidirectional technique described here provides the benefits of both unidirectional techniques (mobility) and approaches, which leave most attachments intact (multidirectional blood flow) without the compromised blood flow and mobility that these approaches, respectively, impose.
REFERENCES
Jean-Claude Schwartz, MD, PhD – Breast Cancer and Reconstructive Surgeon

As a national leader in oncoplastic breast surgery, Dr. Schwartz has developed and published some of the most innovative ways to save women’s breasts, allowing them to avoid mastectomy and multiple reconstructive surgeries. Dr. Schwartz’s techniques have benefitted his patients and other surgeons around the world.
There is no breast surgical oncologist in the United States that offers the same variety of oncoplastic options, nor has contributed more to the different fields of breast reconstruction than Dr. Schwartz.