Fax: 770-339-9804
Lawrenceville, Georgia 30046

Originally published on PRS Global Open website in October 2020
Abstract
Background:
Postmastectomy reconstruction in obese patients has a significant risk of complications and poor outcomes after implant-based and autologous methods. Here we present 22 consecutive patients with Class III obesity [body mass index (BMI) > 40 kg/m2] who underwent reconstruction with a muscle-sparing latissimus dorsi (MSLD) flap.
Methods:
A chart review of a single surgeon experience with 22 consecutive patients with Class III obesity who underwent postmastectomy reconstruction with an MSLD flap was performed. Demographics, operative details, outcomes, and complications were evaluated.
Results:
Twenty-two patients underwent 29 mastectomy and MSLD reconstructions. There were no flap failures. The average BMI was 47.2 kg/m2, including 12 patients with BMI > 50 kg/m2. Seven breasts demonstrated partial nipple and or mastectomy flap necrosis. There was 1 (3.4%) donor site dehiscence that healed with outpatient wound care and 1 (3.4%) seroma that required multiple aspirations in the office. The average operative times were 178 and 420 minutes for unilateral and bilateral mastectomy and immediate reconstructions, respectively. The average hospital length of stay was 0.56 and 1.3 days for unilateral and bilateral surgeries, respectively.
Conclusions:
These results demonstrate the utility of the MSLD flap in reconstructing the very obese. Operative times and lengths of stay compare favorably with conventional latissimus dorsi flap and abdominal-based microvascular free tissue transfer reconstructions. While our complication rates were higher than historically seen for patients with normal BMIs, there were no instances of flap failure, making this a viable reconstructive option for these very high-risk patients.
INTRODUCTION
Postmastectomy reconstruction in patients with Class III obesity or greater poses significant challenges to the surgeon regardless of modality.1,2 These patients have higher rates of reconstructive failure with prosthetics, and their aesthetic results using an implant-based approach are typically inferior to those patients in the normal body mass index (BMI) ranges.3,4 Higher rates of reconstructive failure using implants can be secondary to poorer wound healing of the mastectomy flaps, higher rates of seroma formation, and increased dead space after surgery.3 Poor aesthetic results can result from the a limited size range available for implants, as their body habitus dwarfs the largest implants available. In addition, these patients often have an abundance of lateral chest wall adipose tissue after mastectomy that detracts from an implant-based reconstruction, as the transition between the implant and this excess tissue is abrupt and unnatural. Implant-based approaches are especially challenging in a patient who demands a unilateral mastectomy and reconstruction, as the often large and ptotic contralateral breast cannot be reduced and reshaped in a reliable fashion to match a prosthetic reconstruction.5 The best outcomes in these very obese patients, especially for unilateral reconstructions, is to proceed with an autologous approach.4,5
Traditional autologous reconstructions in these patients rely on abdominally based free flaps, where an abundance of soft tissue is available to reconstruct one or both breasts.6 Microvascular deep inferior epigastric (DIEP) flaps have been described in obese patients with higher than average complication rates.7 In general, the BMI limit for these approaches has been ≈40 kg/m2 to avoid prohibitively high complications rates.8 Recent reports with abdominally based microvascular free flaps still document significant donor site complications in obese patient populations ranging from 5% to 60%.1,3,4,9–14
In addition, there is a more significant rate of fat necrosis and flap failure in these patients not observed in those with BMIs in the normal range.1–4,6 Recent reports document operative times between 450 and 700 minutes for unilateral and bilateral reconstructions (not including the oncological resection) in these patient populations, respectively.9,15 Patients with Class III obesity often have multiple other comorbidities, and the safety of these prolonged operative times may raise significant concerns on the part of both surgeons and patients. Given the prolonged operative times and potential for risk for perioperative complications requiring unplanned additional surgery and or hospitalizations, the costs of this approach are not insignificant.16–18
The latissimus dorsi (LD) myocutaneous flap has been previously described by others as a definitive autologous reconstructive option in some patients with lower complication rates than abdominal free tissue transfer, especially in patients who are obese.9,19,20 The major criticism of this flap is the lack of sufficient volume to provide a definitive reconstruction, which is often addressed with both immediate21–24 and or delayed fat grafting,25 implant placement26–28 or harvesting an extended flap with additional soft tissue from the donor site.29,30 The muscle-sparing latissimus dorsi (MSLD) flap has also been described as an alternative to the traditional LD flap for total or partial breast reconstruction, with the advantages of ease of dissection (when compared with a perforator flap), preservation of 75% of the muscle with no obvious postoperative functional sequelae with regard to strength and range of motion, significantly decreased incidence of donor site seroma, less postoperative pain and quicker recovery, preservation of the axillary silhouette with a minimal risk of contour deformities in the back.31–35
The safety and efficacy of the MSLD flap for total breast reconstruction patients with class III obesity has not been previously described. We have found that in patients with a BMI > 40 kg/m2, the MSLD does not require volume supplementation or extensive donor site dissection, as these patients have excessive fatty deposits to provide for a safe, single-stage definitive postmastectomy reconstruction in either the immediate or delayed setting. Here we describe our experience using the MSLD flap to reconstruct patients with class III obesity after nipple-sparing mastectomy or Wise-pattern mastectomy and free nipple grafts.
METHODS
A retrospective chart review of all consecutive postmastectomy MSLD reconstructions was performed for all patients with a BMI > 40 kg/m2 from July 2018 to April 2020, who had a minimum of 3-month follow-up. All patients were seen by a specialist plastic surgeon and either were refused a free flap surgery or decided against proceeding with a microvascular reconstruction. The mastectomy and reconstruction were then performed by the author.
Demographic information (including age and BMI) was recorded. Comorbidities assessed included both hypertension and diabetes requiring medical treatment. Active smokers and diabetics with hemoglobin A1c > 7.0 were not offered reconstructive surgery. There were no patients included in this series who had a history of radiotherapy. Timing of reconstruction was characterized as immediate or delayed. Oncological treatments (including neoadjuvant or adjuvant chemotherapy and postmastectomy radiotherapy) were also recorded. The incidence of complications was evaluated by a chart review and recorded as minor or major for each breast and donor site. Minor complications included those that could be handled in the outpatient setting, including wound care for the mastectomy or donor site, antibiotics for cellulitis, or seroma aspiration. Major complications required general anesthesia for hematoma evacuation, debridement of the flap, mastectomy wound revision, or donor site complications. Additional data collected included operative times, mastectomy weights, extent of axillary surgery, unilateral versus bilateral reconstructions, nipple-sparing versus skin-reducing Wise-pattern technique, the length of time drains were left in situ, the frequency of revisions, and hospital length of stay.
Surgical Technique
The MSLD flap was harvested as previously described by others31–35 except for the following modification that all surgeries started in the lateral decubitus position to raise the MSLD flap followed by mastectomy or flap inset in the supine position (Fig. 1). This allowed for unilateral and bilateral mastectomy and reconstructions with only 1 or 2 position changes, respectively. We designed the skin paddle to lie within the natural skin creases of the back to safely maximize the size and volume of the flap. The donor sites were closed with quilting sutures, and a single drain was left in place until the output was less than 30 mL for 2 consecutive days. The mastectomy site was also drained in a similar fashion. The viability of the mastectomy and MSLD flaps were examined by evaluating tissue oxygenation saturation via near infrared spectroscopy using SnapshotNIR (Kent Imaging, Calgary, AB, Canada) and debrided as necessary. Immediate reconstructions involve either nipple sparing procedures through inframammary incisions in continuity with the donor site or Wise-pattern skin reduction incisions with free nipple grafts, which were also in continuity with the donor site. All delayed reconstructions were performed after first-stage Goldilocks mastectomy with free nipple grafts, as previously described36 at a minimum of 6 months postoperative. Additional representative patients are presented in Figures 2–4.
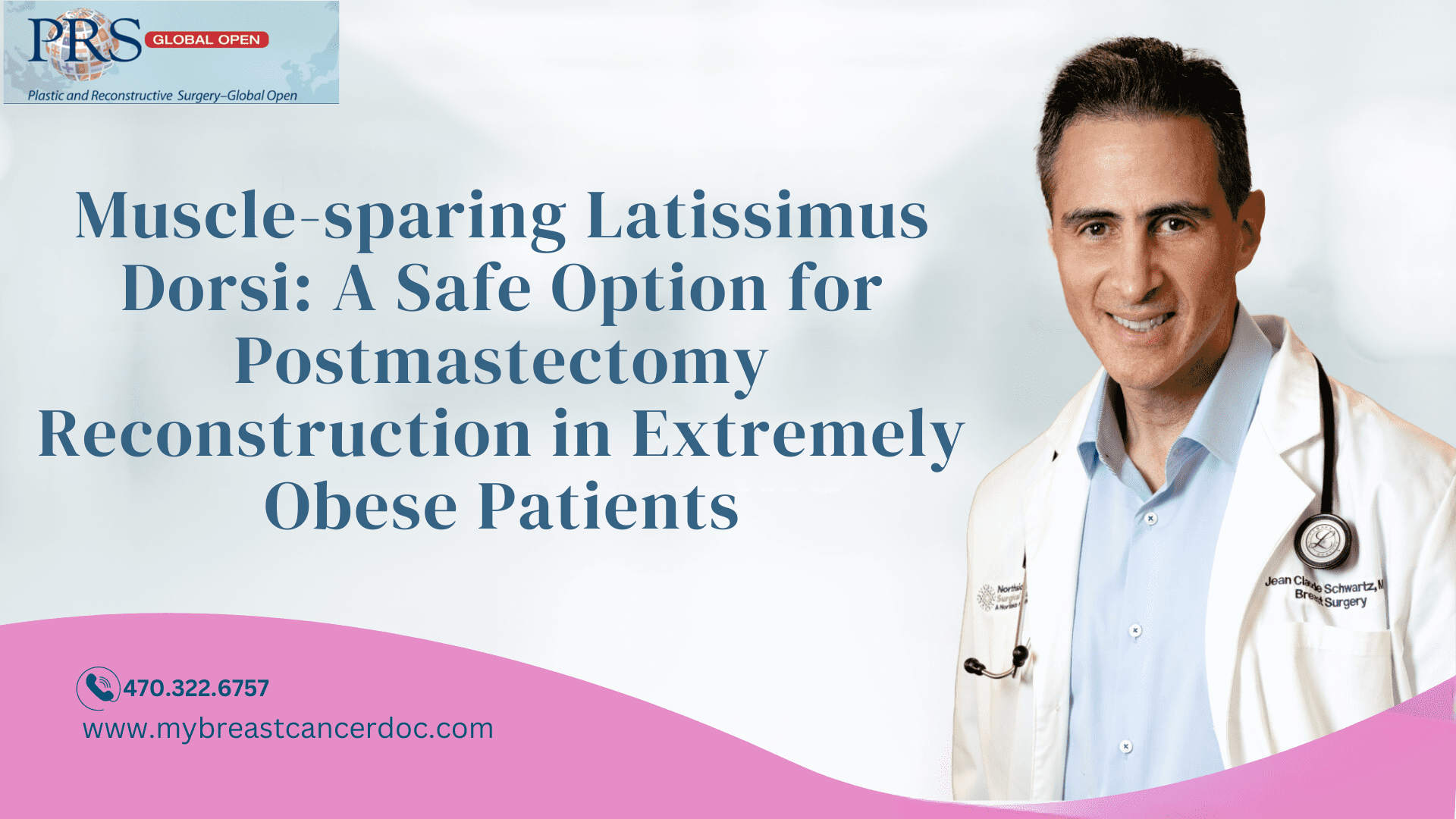
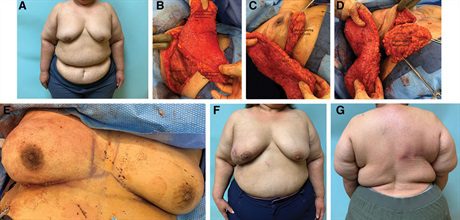 Fig. 1.:
Fig. 1.:
A 50-year-old woman with 8 cm of right breast ductal carcinoma in situ who required a mastectomy. She had a BMI of 51.2 kg/m2 (A) and desired to undergo an autologous reconstruction. She has sufficient lateral chest wall and posterior back adipose tissue supported by the MSLD to proceed with a single-stage autologous reconstruction. She is first placed in the lateral decubitus position, where the MSLD is de-epithelialized and then raised (B). Only 25% of the muscle is required to support the flap with the remainder left undisturbed (B). The patient is then turned supine, where the nipple-sparing mastectomy and lymph node dissection is performed (C and D). We often perform the mastectomy through an inframammary incision, which lies in continuity with incision used to raise the MSLD. Alternatively, a Wise-pattern mastectomy with free nipple grafts can also be performed in patients with extensive ptosis. The flap is then positioned and secured to the chest wall with multiple absorbable sutures, leaving an immediately pleasing aesthetic result (E). This surgery is routinely performed in less than 3 hours, often without hospital admission. This patient suffered significant mastectomy flap necrosis, which healed over the course of 10 weeks with outpatient wound care (F). Her donor site healed without difficulty (G).
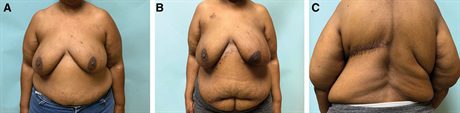 Fig. 2.:
Fig. 2.:
A 61-year-old woman with multicentric left breast cancer who required a mastectomy and desired to undergo an immediate reconstruction. She had a BMI of 46.7 kg/m2 (A). Given her body habitus and breast size coupled with her desire for unilateral surgery, she is best served with an autologous reconstruction. She is shown 8 months after her mastectomy and after the immediate reconstruction (B). Her donor site heals without incident (C).
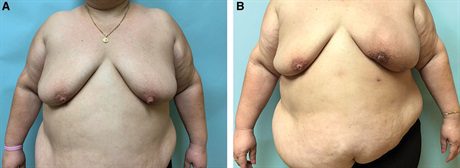 Fig. 3.:
Fig. 3.:
A 45-year-old woman with a BMI of 52.2 kg/m2 and extensive invasive lobular carcinoma of left breast who required a mastectomy (A). She underwent mastectomy and immediate MSLD reconstruction in 170 minutes and is discharged that same day. She had some delayed healing of her mastectomy flap, which healed with outpatient wound care within 6 weeks. Her final pathology revealed multiple positive lymph nodes, which were not detected on preoperative imaging or on an intraoperative frozen section analysis and therefore requires postmastectomy radiotherapy. She is shown 8 months after the completion of radiation therapy (B).
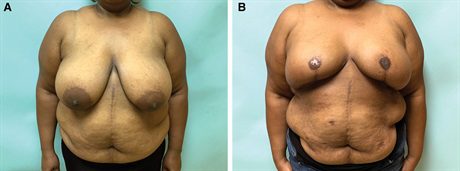 Fig. 4.:
Fig. 4.:
A 41-year-old woman with right breast cancer and a BMI of 42.1 kg/m2 desires bilateral mastectomy and immediate autologous reconstruction (A). She is shown 12 months after bilateral Goldilocks mastectomy with free nipple grafts and immediate MSLD reconstruction (B). She underwent subsequent fat transfer to the right breast for improved symmetry (not shown).
Statistical Analysis
Descriptive statistics were used to describe the sample characteristics. Categorical variables were evaluated with frequencies and percentages, while continuous variables had their means, medians, SDs, and ranges calculated.
RESULTS
Patient demographics, comorbidities, and oncological treatment details are provided in Table 1. In total, 29 breasts in 22 patients with class III obesity met inclusion criteria for the study. The mean age of the population was 53.2 years (range, 36–71 years), and mean BMI was 46.7 kg/m2 (range, 40.7–54.2 kg/m2). There were no patients in this series with a history of previous radiotherapy. Four breasts did undergo radiation therapy after flap reconstruction. Procedural details are provided in Table 2. Twenty-one flaps were used for immediate reconstruction and 8 flaps were used for delayed reconstruction. Of the 21 breasts that underwent immediate reconstruction, 16 were nipple-sparing procedures and 4 were Wise-pattern skin-reducing mastectomies with free nipple grafts (1 was a skin-sparing mastectomy). Operative times were 178 (range, 158–240 minutes) and 420 (range, 387–587 minutes) minutes for unilateral and bilateral mastectomy and immediate flap reconstruction, respectively. For delayed cases, operative times were 122 (range, 102–151 minutes) and 272 (range, 201–347 minutes) minutes for unilateral and bilateral cases, respectively. The average hospital length of stay was 0.56 (range, 0–2 days) and 1.3 (range, 1–4 days) days for unilateral and bilateral surgeries, respectively. The average mastectomy weight was 1157 g (range, 677–1562 g). The average time was 12.2 days (range, 6–22 days) for all drains to be removed. No patients have required or requested implant placement. No patients have thus far requested revision of their donor sites, and 1 patient (3.4%) has requested revision with fat transfer. Average follow-up time is 10.2 months (range, 3–13 months), with a minimum of 3 months. Minor and major complications are presented in Table 3. There were no flap losses or major complications related to the flap (donor site or flap). No patients required hospital admission for a medical complication. Seven patients had evidence of mastectomy flap necrosis (all after immediate reconstruction), none of which required operative debridement. One breast (3.4%) required immediate readmission and surgery for hematoma evacuation (mastectomy site). Additional minor complications related to the reconstructed breast included wound infection [2 of 29 (6.9%)], fat necrosis [5 of 29 (17.2%)], and seroma [2 of 29 (6.9%)]. With regard to the donor site, there was 1 (3.4%) persistent seroma requiring repeated office aspirations and 1 (3.4%) donor site wound dehiscence requiring outpatient wound care that was completely healed within 4 weeks.
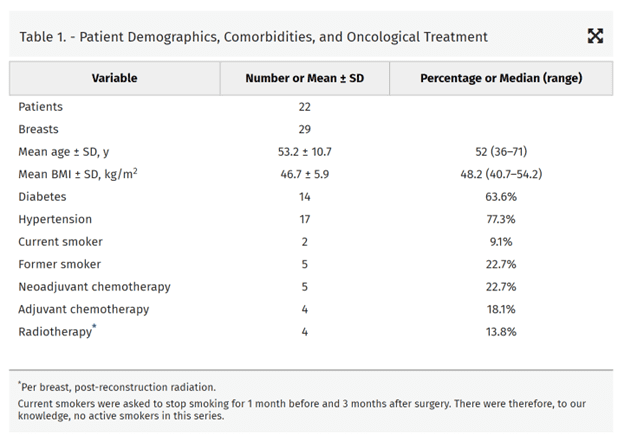

Table 3. – Postoperative Complications
| Complications | Number | Percentage |
| Minor complication, per breast | 17 | 58.6 |
| Skin necrosis (breast) | 7 | 24.1 |
| Seroma (breast) | 2 | 6.9 |
| Infection (breast) | 2 | 6.9 |
| Donor site | 2 | 6.9 |
| Fat necrosis | 3 | 10.3 |
| Major complication requiring reoperation, per breast | 1 | 3.4 |
| Donor site | 0 | 0.0 |
| Seroma, breast | 0 | 0.0 |
| Skin flap necrosis | 0 | 3.4 |
| Hematoma | 1 | 3.4 |
| Infection | 0 | 0.0 |
| Flap failure | 0 | 0.0 |
DISCUSSION
Breast reconstruction after mastectomy surgery is most commonly performed with prosthetic devices.37 The largest silicone implants available often do not provide sufficient volume to reconstruct women with class III obesity or greater.38 In addition, for patients with a BMI> 40 kg/m2, several studies have documented prohibitive rates of complications, including infections and reconstructive failure using implant-based methods.1–3 Aesthetic results in this group of patients have also been documented to be poor with especially unacceptable results and lower patient satisfaction after unilateral reconstructions.3–5 This makes intuitive sense, as patients with this type of body habitus do not have contralateral healthy breasts that can be reliably reshaped into breasts that mimic the contralateral implant-based reconstruction. Hybrid postmastectomy reconstructions using combined autologous and implant-based methods can often lead to improved symmetry.39,40 In these cases, the contralateral breast can also undergo reduction and augmentation to obtain even better results.
Given the unreliable and poor results that morbidly and super obese patients have after implant-based reconstructions, several surgeons prefer autologous approaches in these patients.4,6,8,12,15 The most traditional approach would be an abdominally based tissue reconstruction, previously a transverse rectus abdominis myocutaneous flap, but now more commonly a DIEP flap. Although the newer microvascular methods have decreased donor site morbidity with regard to abdominal wall hernias, wound healing complications are still prevalent and occur with increased frequency in the very obese.1–3,6–8,10–15 Recently, a report demonstrated a combined 36% readmission rate for medical or surgical complications for patients with an average BMI of 35 kg/m2 after free tissue transfer breast reconstruction.9 Microvascular reconstructions require surgical expertise that is not readily available at all institutions. They require prolonged operative times that can require up to 10 hours for bilateral cases (not including the mastectomy surgery).9 These extended operative times utilize significant healthcare resources and put these patients, many of whom have additional comorbidities, at risk for additional medical complications.18 There is a frequent requirement for donor site revisions for scar cosmesis after poor wound healing, which is far less common for the back scar after LD or MSLD harvest.9,27–33 Additionally, as these surgeries utilize both lower abdominal sites, they make a future contralateral abdominally based reconstruction, for patients only committing to upfront unilateral surgery, impossible. This necessitates a significant consideration for upfront bilateral surgery if the patient may desire or require contralateral mastectomy and reconstruction in the future.
While the LD flap has been well described as a simpler autologous alternative to free tissue transfer,19–25 critics maintain that it seldom provides enough volume on its own for a definitive reconstruction. As patients’ BMIs rise to the class III obesity range and beyond, they have sufficient local fatty deposits near and around their LD muscle to provide a definitive reconstruction without resorting to an implant, extensive local soft tissue harvest, or immediate fat transfer, which is often not the case in patients who are less obese (BMI < 40 kg/m2). In addition, by using the MSLD approach, they have minimal donor site morbidity (comparable to a thoracodorsal artery perforator flap), negligible rates of seroma, no postoperative shoulder dysfunction, less pain, and a quicker recovery than seen with the conventional LD.31–35 This is in stark contrast to the donor site complications these patients would have encountered after an abdominally based reconstruction. In our experience, MSLD flaps in this BMI range do not frequently suffer from significant clinically obvious fat necrosis and we have no instances of flap failure that required debridement. The MSLD provides a soft, natural result that gives a good symmetry with the contralateral native breast. (See Video 1 [online], which displays 1 year postoperative result for the patient presented in Figure 2.)
Video 1.
Video 1 from “Muscle-sparing latissimus dorsi: a safe option for post-mastectomy reconstruction in the extremely obese”
In our hands, a unilateral mastectomy (nipple sparing or Wise-pattern with free nipple graft) and immediate reconstruction takes less than 3 hours. We accomplish this by starting in the lateral decubitus position to harvest the flap and then turning supine to perform the mastectomy and then position the flap, requiring only 1 position change. Nearly half of our patients requiring unilateral surgery were operated on in our ambulatory surgery center, with discharge on the day of surgery, while the others underwent surgery in the hospital with 23 hour observation. Bilateral mastectomy and reconstruction cases take nearly 7 hours, again raising both flaps first in their respective lateral decubitus positions and then turning supine for the mastectomy and reconstruction, requiring only 2 position changes. These operative times compare very favorably with those required for bilateral LD surgery with fat transfer or bilateral DIEP surgery, which can take 8 and 10 hours, respectively, in the delayed setting after the mastectomy has been previously performed.9 The MSLD flap does not require a decision on the patient’s part to commit to a bilateral surgery. Unlike the DIEP flap, the contralateral donor site is preserved with the MSLD and the patient can undergo a unilateral mastectomy and reconstruction without concerns that she will not have a future autologous option that will provide her a good symmetry with her current reconstruction. Unlike the DIEP flap, all reconstructive breast surgeons should be capable of performing an MSLD. Most patients in the morbidly and super morbidly obese range benefit from the body contouring provided by the MSLD as this flap is predominantly composed of the excess lateral chest wall fat and skin, which is typically present in vast abundance. In these women who have small breast in relation to their larger body habitus, the MSLD flap is often significantly larger than their breasts. However, these tissues would likely require a direct excision to optimize an implant-based or abdominal free flap reconstruction.
A recent report by Novak et al9 compared their experience with the fat grafted latissimus versus free tissue transfer for postmastectomy reconstruction in obese patients. They concluded that in the obese patient population, the fat grafted latissimus provides a completely autologous reconstruction with lower complication rates, decreased operative times, and shorter hospital lengths of stay compared with free tissue transfer. We are in agreement with these conclusions and further contend that the MSLD can safely reconstruct these obese patients with less morbidity than utilizing the full LD. Our results also demonstrate that as BMIs increase (our average BMI was 46.7 kg/m2 when compared with 37.6 in the report by Novak et al9), patients do not require immediate fat grafting. We also demonstrate the safety of immediately reconstructing these patients in less than 3 hours for a unilateral reconstruction by starting in the lateral decubitus position (the report by Novak et al9 documented predominantly delayed reconstructions with average operative times of 294 minutes for a unilateral procedure that did not include the mastectomy). We believe that the MSLD approach with decreased operative times, decreased hospital length of stay, quicker recovery, less morbidity, and fewer required surgical procedures (than either a full LD or free tissue transfer) will encourage more surgeons and obese patients to pursue immediate autologous reconstruction.
There were no readmissions in our series for major medical complications and 1 readmission to surgically evacuate a postoperative hematoma despite an average BMI of 46.7 kg/m2. While only 1 patient (3.4%) has thus far requested revision of her reconstruction with fat transfer, expectations in this patient population may not be comparable to those in patients with lower BMIs. The most significant complication in our series was not related to the MSLD flap or donor site, but mastectomy flap necrosis, all of which occurred after immediate reconstruction. This had led us to strongly consider an initial Goldilocks mastectomy with free nipple grafts followed by delayed MSLD reconstruction in our highest risk patients. This typically requires a 3-month waiting period to allow for the tissues to heal before flap placement. This strategy is useful in patients who require radiotherapy—optimizing the skin envelope with a Goldilocks procedure and then delaying flap placement for a minimum of 6 months after radiation is completed. In higher-risk patients that do not wish to wait for several months to complete the reconstructive process, we will proceed with mastectomy through lateral Wise incisions41 followed by a flap reconstruction and Wise-pattern closure with pedicled nipple repositioning versus free nipple graft 14–21 days later.
CONCLUSIONS
With increasing BMIs into the class III obesity range and beyond, the MSLD flap can provide an immediate definitive reconstruction after mastectomy without the use of an implant, extensive donor site dissection, or immediate fat transfer. Patients have minimal donor site morbidity, and the flap viability is excellent. Unilateral mastectomy and immediate MSLD reconstruction can be performed in less than 3 hours as an outpatient procedure, with the same-day discharge. The most common complications are related to mastectomy flap healing. We believe the MSLD is the preferred reconstructive option for patients in these extreme BMI ranges.
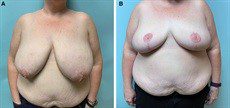 Fig. 5.:
Fig. 5.:
A 41-year-old woman with a BMI of 42.1 kg/m2 who required right breast cancer bilateral mastectomy and immediate autologous reconstruction. She is shown 12 months after bilateral Goldilocks mastectomy with free nipple grafts and immediate MSLD reconstruction. She underwent fat transfer to the right breast for an improved symmetry.
REFERENCES
Jean-Claude Schwartz, MD, PhD – Breast Cancer and Reconstructive Surgeon

As a national leader in oncoplastic breast surgery, Dr. Schwartz has developed and published some of the most innovative ways to save women’s breasts, allowing them to avoid mastectomy and multiple reconstructive surgeries. Dr. Schwartz’s techniques have benefitted his patients and other surgeons around the world.
There is no breast surgical oncologist in the United States that offers the same variety of oncoplastic options, nor has contributed more to the different fields of breast reconstruction than Dr. Schwartz.