Fax: 770-339-9804
Lawrenceville, Georgia 30046


Originally published on PRS Global Open website in October 2018
Oncoplastic breast-conserving surgery describes a set of techniques that allow for generous oncological resection with immediate tumor-specific reconstruction. These techniques are classically divided into either volume displacement (local breast flaps and or reduction mammaplasty/mastopexy strategies) versus volume replacement strategies (transfer of autologous nonbreast tissue from a local or distant site and, less commonly, implant placement). There have been few descriptions of merging these 2 classical approaches to facilitate breast-conserving surgery. The purpose of this report was to evaluate the efficacy of combining the most common oncoplastic volume displacement strategy (Wise pattern mammaplasty) with simultaneous autologous volume replacement from the lateral intercostal artery perforator (LICAP) flap to reconstruct the extensive partial mastectomy defect in patients with ptosis.
A retrospective analysis of 25 consecutive patients with multifocal or multicentric breast cancers who underwent simultaneous volume replacement from the LICAP flap and volume displacement (Wise pattern mammaplasty) to achieve breast conservation was performed between January 2016 and January 2018. Clinical outcomes and postoperative complications were examined.
Twenty-five consecutive patients with a mean age of 56 years (range, 37–74 years) and mean body mass index of 28 kg/m2 (range, 22–37 kg/m2) all successfully underwent breast conservation by simultaneously employing the LICAP flap and Wise pattern mammaplasty to reconstruct the partial mastectomy defect. The average resection specimen weight was 220 g (range, 130–310 g) and average size of the malignancy resected was 6.5 cm (range, 3.7–9.2 cm). Three patients (12%) required re-excision for close or positive margins but were ultimately cleared. There were no complications related to the donor site. There were 4 patients (16%) with delayed wound healing related to the Wise pattern closure but no instances of LICAP necrosis or failure.
The merger of Wise pattern volume displacement and autologous volume replacement techniques represents a novel strategy that is useful in the most challenging breast conservation patients with some degree of ptosis.
Breast conservation surgery is used in 60–70% of breast cancer patients in the western world with documented quality of life, aesthetic, functional, and possible oncological benefits compared with mastectomy.1,2 Unfortunately, long-lasting deformities after breast conservation are not uncommon.3 Significant deformity reliably occurs when more than 20% of the breast is removed regardless of the skill of the breast surgeon. Oncoplastic volume displacement strategies were devised to immediately repair partial mastectomy defects and avoid deformity. Clough described level 1 techniques that mainly comprised local glandular breast flaps that are mobilized to fill the lumpectomy defect when less than 20% of the breast is resected.4 He further described level 2 techniques that revolved around mammaplasty strategies when more than 20% of the breast is removed.4 Level 2 techniques are typically only possible in patients with some degree of ptosis. The Wise pattern mammaplasty is the most common and versatile level 2 approach. Further refinements of level II techniques have been described, which involve extended pedicles and other secondary pedicles to help reshape the breast and fill large defects located outside the standard Wise pattern.5 Silverstein et al.6 have additionally pioneered “Extreme Oncoplasty” for the most challenging cases of breast conservation involving multifocal or multicentric breast cancers utilizing complex breast reshaping strategies and the “split reduction” when the overlying skin must be sacrificed to obtain clear margins.6,7
Alternatively, in the patient with small breasts and minimal or no ptosis, autologous volume replacement techniques are often required for breast conservation when a significant percentage of the gland is removed. These strategies include, among others, the traditional latissimus dorsi or mini-flap and the various chest wall pedicled perforator flaps including the thoracodorsal artery, lateral intercostal artery perforator (LICAP), serratus anterior, and anterior intercostal perforator flaps.8 Strategies here typically do not involve contralateral symmetry procedures as the breast volume that is removed is simply replaced with a local flap with no nipple or skin repositioning as the goal here is to leave the breast looking as it did before surgery.
Despite the lack of significant precedent, volume replacement and displacement strategies can be performed simultaneously. Barnea et al.9 and Nahabedian10 have independently described the combination of prosthetic volume replacement with very basic volume displacement maneuvers.10 These strategies may have limited utility in the patient with an extensive defect that results in an irregular contour deformity that is best filled with autologous tissue that can be precisely fit into the defect. Prosthetic volume replacement strategies have the additional drawback of placing an implant into a field that will require radiotherapy. This approach is also challenging in patients with significant ptosis, as this would require an augmentation and aggressive mastopexy in a patient who also requires a simultaneous cancer resection and lymph node dissection.
In those patients with extensive cancers and grades 1–3 ptosis that desire breast conservation, level II mammaplasty strategies may not be sufficient to reconstruct the breast and avoid deformity if there is not enough residual breast volume after cancer resection. We have found that these patients benefit from both a Wise pattern mammaplasty volume displacement approach in concert with volume replacement from the LICAP flap. Here, we present a series of 25 consecutive patients who underwent immediate, simultaneous Wise pattern mammaplasty reconstruction with additional volume supplementation from the LICAP flap after partial mastectomy. Although the Wise pattern mammaplasty in concert with the LICAP flap has never been previously reported in the breast cancer reconstruction patient, it has been well described in the massive weight loss patient.11–13 The purpose of this study was to determine the efficacy of this strategy in the ptotic patient with an extensive breast cancer that otherwise might not be amenable to breast conservation.
Twenty-five consecutive patients who underwent simultaneous LICAP reconstruction and a Wise pattern mammaplasty for reconstruction of the partial mastectomy defect between January 2016 and January 2018 were included in the study. Twenty-four patients also underwent contralateral Wise pattern mammaplasty for symmetry. Active smokers were excluded from consideration unless they abstained from nicotine products for 30 days before surgery. Demographics, comorbidities, and details of all surgical procedures were collected through review of the electronic medical records including age, diabetic and smoking status and sternal notch to nipple distances, partial mastectomy weight, size of cancer resected, flap dimensions, rates of close or positive margins requiring re-excision, need for adjuvant chemotherapy or radiotherapy, elapsed time from reconstruction to complete healing, and results of mammagraphic follow-up. Records were also reviewed for the following complications: fat necrosis (defined as a palpable hardening in the reconstructed breast), rates of nipple and LICAP flap necrosis and skin necrosis resulting in delayed wound healing of greater than 4 weeks and donor-site seromas and wound dehiscence.
All continuous variables had their mean values and SDs reported.
Patients were evaluated by the author and determined to be candidates for oncoplastic reduction and contralateral symmetry surgery. In those cases where the author felt volume displacement would not be sufficient to address the partial mastectomy deficit, a LICAP flap was added to the procedure as described previously,14 with representative patient markings demonstrated in Figure 1A–C. Patients were marked in the standing position the day before surgery with the standard Wise pattern and additional markings to include the LICAP flap as described previously for the massive weight loss patient.11–13 The surgery started in the lateral decubitus position as if we were preparing a latissimus flap. Full-thickness incisions were made through skin and fat until we reached the underlying trapezius and latissimus fascia, which was included in the flap. The flaps had an average height of 8 cm and length of 20 cm depending on pinch thickness and body habitus. The donor sites were then closed in 3 layers and the flap transferred to a sterile bag while repositioning the patient supine. The superior border of the flap was then brought into continuity with the most lateral portion of the Wise pattern as previously described.11–13 Full-thickness incisions for the inferior portion of the flap (at the inframammary fold [IMF]) stopped at the posterior axillary line/anterior border of the latissimus where the perforators begin to arise—this was the pivot point of the flap which could be rotated 180 degrees without difficulty (Fig. 1B). We kept a 6 cm pedicle here to ensure flap viability although typically the entire IMF, and surrounding tissues were never violated unless mandated for oncological reasons. At this point, the Wise pattern was incised, and we used well described techniques to proceed with a medial, inferior, lateral, or superior pedicle oncoplastic reduction depending on tumor location. Sentinel node biopsy was performed through the Wise incisions or through a separate axillary incision. We obtained radiological, gross, and frozen section pathological confirmation of successful extirpation of the cancer and then proceeded with reconstruction. The LICAP flap was de-epithelialized and rotated into the deficit and sutured to the surrounding breast tissue and pectoralis muscle (Figs. 1, 2). Excess flap was resected if the additional volume would result in significant size asymmetry with the contralateral side. The distal tip of the flap was evaluated and resected until reliable arterial bleeding was observed. The flap was positioned in a fashion to simultaneously address the partial mastectomy defect and to best augment the breast volume on an individual basis (Figs. 1, 2). After the defect was reconstructed and the flap secured, the breast was closed using the standard Wise pattern. In all but one case, a contralateral mammaplasty was performed to match the reconstructed breast. We use one drain at the donor site (15 round Blake) and one drain in the reconstructed breast around the flap. We usually do not leave a drain in the contralateral reduction. We do not experience persistent drainage at the donor site as is commonly seen with latissimus flaps. Both drains are routinely removed between postoperative days 3 and 5.
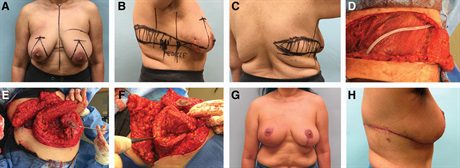 Fig. 1.:
Fig. 1.:
A 55-year-old female with 8 cm of ductal carcinoma in situ in the right upper outer quadrant. She has been recommended to proceed with a skin-sparing mastectomy and reconstruction by another surgical team. She refuses to lose her nipple or to proceed with mastectomy. Her right breast is smaller than her left breast that makes this an even more challenging case. Given her grade 2 ptosis and excess lateral chest wall adiposity, we offer her on oncoplastic reduction in concert with a LICAP flap and demonstrate the markings (A–C). The 3 wires are placed preoperatively by radiology to bracket the area of disease (B and C). The pedicle for the LICAP flap is outlined and typically lies anterior to the latissimus dorsi muscles at the IMF and has a 6 cm base (B). This is the 180-degree pivot point of the flap. The pedicle is more accurately determined intraoperatively and can be confirmed with a unidirectional Doppler. The LICAP flap dissection can be oriented parallel to the IMF to the posterior midline or it can curve upward toward the scapular tip depending on where the excess subcutaneous tissue is most abundant. D, The donor site is seen after raising the flap. The patient is in lateral decubitus position and drain is overlying the latissimus muscle with the incision approaching the posterior midline. The dissection of the inferior portion of the flap stops near the anterior border of the latissimus where the perforators arise. The upper outer quadrant of the right breast (260 g) of breast tissue is resected and specimen mammagraphy confirms successful extirpation of the 8 cm expanse of calcifications. The LICAP flap is easily rotated into the defect to reconstruct the upper outer quadrant of the right breast with internal sutures and to the underlying pectoralis major muscle (E and F). G, Her result, 9 months after radiotherapy is seen. Interestingly, the right breast, which was initially smaller than the left breast, is now slightly larger. This result in only possible by employing both volume replacement and displacement strategies. H, Her postoperative donor site scar is shown. Her scar runs in the bra line but in some patients, we curve the incision cranially toward the superior thoracic spine to harvest more tissue.
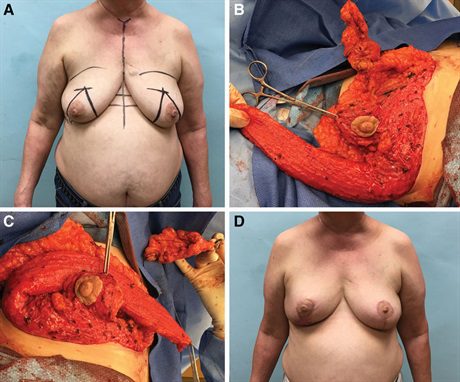 Fig. 2.:
Fig. 2.:
A 60-year-old female with 3 foci of upper inner quadrant right breast cancer spanning 6 cm (A). She underwent neoadjuvant chemotherapy and had a complete imaging response. Despite this, she was recommended to proceed with mastectomy by her local surgeon. She was motivated to pursue breast conservation and sought a second opinion. We felt it was prudent to resect the entire area of previous disease en bloc but realized this would leave her with a large upper inner quadrant deficit. The Wise pattern mammaplasty approach would help but would not be sufficient to avoid deformity. We added the LICAP flap to aid with volume supplementation. After the LICAP flap is raised in the lateral decubitus position, the patient is turned supine, and the partial mastectomy is performed (B). The LICAP flap easily reaches the upper inner-quadrant lumpectomy cavity to help close the partial mastectomy defect (C) and the tip of the flap reaches past the midline. The nipple is supported on an inferior pedicle and the breast is closed using the standard Wise pattern (C). The patient is shown 12 months after the completion of radiotherapy with no evidence of deformity and good symmetry (D).
Twenty-five consecutive patients with a mean age of 56 (range, 37–74 years; SD, 2.1 years) who under underwent combination Wise pattern mammaplasty and LICAP reconstruction with a minimum of 6 months follow-up after radiotherapy. The mean size of the malignancy resected, or extent of disease was 6.5 cm (range, 3.7–9.2 cm; SD, 1.3 cm) on final pathology. The mean partial mastectomy specimen weighed 220 g (range, 130–310 g; SD, 45 g) including the weight from additional margins that were immediately resected at the time of surgery if indicated after consultation with radiology and or pathology. We also recorded sternal notch to nipple distances (mean, 31 cm; range, 23–36 cm; SD, 3.4 cm), body mass index (mean, 28 kg/m2; range, 22–37 kg/m2; SD, 2.7 kg/m2), dimensions of the perforator flap average (mean length, 22 cm; range of lengths, 17–29 cm; SD, 2.1 cm), mean height (7 cm; range of heights, 5–10 cm; SD, 1.3 cm). Six patients had a formal diagnosis of diabetes mellitus, and all healed without complication. Three active smokers were included in our series who successfully abstained from smoking before surgery, all of whom healed without incident.
Three patients (12%) had close or positive margins that were successfully re-excised. Seven patients (28%) underwent preoperative chemotherapy, and 6 patients (24%) underwent adjuvant chemotherapy. All patients underwent adjuvant radiotherapy. There were no instances of nipple necrosis or LICAP flap failure requiring reoperation. There were no persistent areas of fat necrosis related to the LICAP flap on follow-up clinical examination. Six patients (24%) had some fat necrosis related to the pedicle (all inferior pedicles) that was not in the region of the LICAP flap reconstruction. These were noted on clinical examination and were not reported by the patient as bothersome. There were 4 instances (16%) of delayed wound healing secondary to skin necrosis that were conservatively treated related to the Wise pattern closure. All surgical sites were healed by 8 weeks (average, 5.6 weeks; SD, 1.8 weeks). There were no instances of delayed healing of the donor site nor persistent seromas after drain removal.
The average time from reconstruction to radiation was 6.5 weeks (range, 4–10 weeks; SD, 1.5 weeks). The 4 patients that had delayed wound healing of the Wise pattern flaps all started radiotherapy no later than 10 weeks after surgery.
All patients had follow-up mammagraphy performed 6 months after radiotherapy. Twenty patients (80%) had a Breast Imaging-Reporting and Data System score of 1 or 2, whereas 5 patients (20%) had a Breast Imaging-Reporting and Data System 3 score, recommended for 6 months follow-up. These patients had likely benign areas of surgical scarring related to the mammaplasty and or LICAP flap.
We present here a description of the synthesis between volume displacement (oncoplastic reduction/mastopexy techniques) and autologous volume replacement (LICAP flap) to reconstruct the extensive partial mastectomy defect. The only previous description of merging volume replacement and displacement strategies to facilitate breast conservation was by Bornea and Nahabedian who separately described simultaneous placement of a submuscular prosthetic and simple volume displacement maneuvers. These techniques were most applicable to the small breasted nonptotic patient who was not interested in mastectomy or a local flap. Here, we extend the synthesis of volume replacement and displacement strategies to the ptotic patient who is a marginal candidate for breast conservation because of initial breast size and or extent of disease. Additionally, some patients request maintenance or enhancement of breast size after an extensive resection and a supplemental LICAP flap is a safe, simple, and reliable way to accomplish this given the need for future radiotherapy.
Although the approach described here is not well described in the field of reconstruction after breast cancer surgery, it has been extensively studied and reported on in the massive weight loss patient. Wise pattern mastopexy with volume supplementation from LICAP flaps is a well-described approach in the massive weight loss patient to enhance volume and shape of the deflated breast11–13 making use of the excess lateral subcutaneous tissues. We have simply adopted this strategy in the breast cancer patient to allow us to both supplement volume, improve shape, and fill a partial mastectomy defect.
Silverstein et al.6 has described “Extreme Oncoplasty” where sophisticated internal reshaping strategies based on the Wise pattern are employed to facilitate breast conservation in multifocal or multicentric breast cancers that were classically recommended to undergo mastectomy. His results demonstrate aesthetic outcomes and oncological recurrence rates that are very similar to traditional oncoplasty or more standard breast conservation. This gives credence to our ambitious attempts at extreme breast conservation. His group further described the “split reduction” that allows for modification of the Wise pattern to ensure a negative anterior margin by excising skin directly over the tumor and saving inferior breast skin to make up for this deficit.7
Despite these triumphs, there are still a significant number of patients with ptosis but minimal breast volume in comparison to the extent of tissue that requires resection. Despite employing complex internal reshaping strategies, additional volume was required to reconstruct these breasts. In most cases, this was to avoid a deformity. In a few cases, this was to maintain or augment breast volume after resection and was motivated by patient demands. There are numerous advantages to the LICAP flap. It has a reliable blood supply, takes 30 minutes to raise and close the donor site, and does not require microsurgical expertise as the perforators are reliably located within 5 cm of the anterior border of the latissimus dorsi and can be identified easily before surgery with a unidirectional Doppler (although this is not strictly necessary). There is minimal postoperative donor-site pain that heals reliably well. Additional benefits to this approach are that we are not using potentially diseased breast tissue to reconstruct the partial mastectomy defect as is done in traditional oncoplasty. Furthermore, the sophisticated glandular flaps that are created during traditional oncoplasty require aggressive separation of the breast tissue from the skin envelope. In a fatty breast, this can lead to extensive fat necrosis. The LICAP flap is not compromised by the extent of fatty replacement in the breast. Additionally, the extended LICAP flap can reach most parts of the breast and does not compromise the position of the nipple or shape of the new breast mound as the pedicle supporting the NAC and new breast mound can be positioned completely independently of the LICAP flap. In addition, as opposed to traditional oncoplasty, the final breast size is not limited by the residual volume after oncological resection. The LICAP flap allows us to safely augment some patients who would be dissatisfied with their final breast size without the use of an implant in the face of impending radiotherapy. The LICAP flap does not compromise the ability to use the muscular portion of the latissimus flap in the future and allows for the elimination of those lateral chest side rolls of fat that many find bothersome. It is within the skillset of most reconstructive breast surgeons. The use of the LICAP flap in concert with traditional Wise-Pattern oncoplasty is a different approach to standard oncoplastic breast conservation and should be considered in patients with ptosis and a tumor to breast size ratio where deformity might be expected secondary to volume deficiency when employing standard Wise-pattern mammaplasty techniques.
We have refined our technique over the past several years performing this combined procedure. Over time, we realized that de-epithelialization of the flap in situ is more efficient than after raising it and closing the donor site as this is easier if the flap is immobile. We have found that including the muscular fascia of the latissimus and trapezius improves stability and sturdiness of the flap (much like the overlying dermis). We have observed that suturing the flap to the pectoralis can sometimes give an unnatural result with retraction of the tissues by the muscle upon contraction. We prefer to secure the flap to the residual breast tissue. We have discovered that this flap has excellent blood supply and can reach virtually any part of the breast. We have replaced skin that is involved with cancer in the far upper inner quadrant of the breast with skin from the distal tip of the flap without any ischemic compromise.
The oncological resection and the reconstruction here are both performed by the author so there is no difficulty coordinating the schedules of 2 surgeons. Any re-excision that is required occurs quickly after the pathology returns. We feel that delaying re-excision makes it difficult to identify the tissue planes accurately after things scar down. The partial mastectomy cavity, which has been clipped, and has the flap filling it, is easily identified and re-excised in the first 2 weeks after surgery.
We have also gained some insight into obtaining better symmetry between the breasts. We now perform the contralateral mastopexy first before proceeding with the reconstruction of the partial mastectomy defect as this gives us a better idea of what our final volume should be. When we first started, we would often get into the situation where the reconstructed breast was significantly larger than the contralateral skin-only mastopexy that required us to go back and debulk the flap a bit to achieve symmetry. Radiotherapy will result in contraction of the tissues and make the flap side less bulky laterally at the pivot point, so it is wise not to over-correct this. We do routinely debulk the flap to some extent at the pivot point more superficially toward the dermis as the perforators are deep and come through the muscle at the inframammary fold near the anterior border of the latissimus. As we gained more experience with this flap, we felt more comfortable thinning out the flap superficially near the pivot point to make this area less bulky and more similar to the contralateral healthy side. Finally, we always reserve the use of liposuction several months after radiotherapy to debulk the lateral reconstructed breast if we felt it was still too bulky. This could be performed without taking into consideration the location of the perforators as the flap has recruited enough collateral blood flow to survive. We have recently performed bilateral LICAP flaps (not included in the series) and this obviously gives the best symmetry.
This series of patients described were all done in the immediate setting with the use of intraoperative gross and frozen section evaluation to confirm clearance of the cancer before reconstruction. Many of these patients had extensive ductal carcinoma in situ where the likelihood of margin involvement was higher and therefore an aggressive en bloc resection was planned to attempt to clear the disease. Others had extensive cancers before chemotherapy and had complete imaging responses after completion but still required a large segment of tissue removed. Presently, in cases of extensive ductal carcinoma or any significant uncertainty about margin status, we now routinely delay the reconstruction until the final pathology report is available. This allows us to definitively determine the amount of flap volume required to reconstruct the breast deficit and to obtain the best symmetry with the contralateral breast in a second surgery.
Wise pattern oncoplastic techniques have allowed surgeons to remove extensive breast cancers and reconstruct ptotic patients with smaller breasts while avoiding a postoperative deformity. As we extend the oncological indications for these techniques and apply them to even smaller breasted women with larger cancers, we will reach a point where additional volume is required from outside the breast to avoid mastectomy. The LICAP flap can provide a reliable, safe, and simple way to supply this volume and to facilitate breast conservation in an even greater proportion of our patients. Although the simultaneous use of volume displacement and replacement strategies has not been widely described in the literature, we believe it should be strongly considered in any patient who is a marginal candidate for traditional Wise-pattern oncoplastic approaches.
REFERENCES
Jean-Claude Schwartz, MD, PhD – Breast Cancer and Reconstructive Surgeon

As a national leader in oncoplastic breast surgery, Dr. Schwartz has developed and published some of the most innovative ways to save women’s breasts, allowing them to avoid mastectomy and multiple reconstructive surgeries. Dr. Schwartz’s techniques have benefitted his patients and other surgeons around the world.
There is no breast surgical oncologist in the United States that offers the same variety of oncoplastic options, nor has contributed more to the different fields of breast reconstruction than Dr. Schwartz.