Fax: 770-339-9804
Lawrenceville, Georgia 30046

Originally published on PRS Global Open website in December 2015
Abstract
Summary:
Obese patients undergoing mastectomy have been documented to have improved outcomes after autologous reconstruction. The Goldilocks mastectomy (GM) has been proposed as a low-risk first-stage bridge to a second-stage definitive reconstruction. Goldilocks patients who desire autologous reconstruction with significant volume increase must consent to a second-stage flap, which typically requires a donor site with additional scarring and morbidity. We have found that many obese Goldilocks patients have significant excess upper abdominal subcutaneous tissue and are good candidates for a second-stage reverse abdominoplasty, which can provide them a completely autologous reconstruction. This approach adds minimal additional scarring, improves upper abdominal contour, and has a low rate of donor site complications. Here, we present 15 consecutive obese patients who underwent first-stage GM followed by second-stage reverse abdominoplasty, providing them with a completely autologous, low-risk reconstructive option.
Takeaways
Question: How can we provide obese, ptotic patients a low-risk autologous reconstructive option after mastectomy?
Findings: This study demonstrated the safety of using a first-stage Goldilocks mastectomy followed by second-stage reverse abdominoplasty to provide obese, ptotic patients a completely autologous, safe reconstruction after mastectomy.
Meaning: Many high-risk obese, ptotic women who were refused more traditional forms of autologous reconstruction after mastectomy may be good candidates for a Goldilocks mastectomy followed by second-stage reverse abdominoplasty, extending the indications for autologous reconstruction to higher-risk obese patients.
INTRODUCTION
Postmastectomy reconstruction in obese patients demonstrates increased complication rates.1 Although the Goldilocks mastectomy (GM) was initially described for women who refused a breast amputation or formal breast reconstruction,2 it has evolved into a strategy to provide higher-risk women a safe, first-stage surgery in preparation for second-stage surgery,3,4 providing a well-healed, reduced skin envelope.
While implant reconstruction is the predominant method of postmastectomy reconstruction,5 abdominal reconstructions have demonstrated fewer complications and improved satisfaction in obese patients.6 Recently, we have realized that some obese patients have significant upper abdominal tissue and are candidates for reverse abdominoplasty (RA) after first-stage GM.
PATIENTS AND METHODS
We reviewed obese patients [body mass index (BMI) > 30 kg/m2] who underwent unilateral or bilateral GM followed by second-stage RA from January 2021 through May 2023 with at least 6 months follow-up. Active smokers were excluded from consideration of this technique.
The following patient variables, operative details, and complications were recorded: age; BMI; operative duration; patient comorbidities; operative duration; height, width, and thickness of the RA flap; major and minor complications; rate of clinical flap fat necrosis (defined a palpable hardening in the reconstructed breast with at least 6 months follow-up); and length of follow-up.
OPERATIVE TECHNIQUE
At least 6 months after GM,2 an RA was performed,7,8 with the following modifications7,8(Fig. 1). Importantly, the final result relies heavily on a GM performed by the author that maximally preserves subcutaneous tissue. Obese patients were candidates for this technique if they had significant excess upper abdominal tissue and refused implant and/or flap reconstruction. After designing the RA that spanned both breasts below the inframammary folds (IMFs) the skin was deepithelialized. (Fig. 2) We then created two autologous breast implants by dividing the RA in the midline. An 8-cm dermal attachment was left at the base of each flap, centered below the meridian. [See Video 1 (online), which displays a second-stage reverse abdominoplasty surgery performed after first-stage GM. This video demonstrates creation of the left breast “autologous implant” from excess tissue below the inframammary fold.] [See Video 2 (online), which displays a reverse abdominoplasty used to augment the left breast after GM and flap reconstruction. Here, we demonstrate how far we must undermine—down to the umbilicus, but making sure to spare the periumbilical perforators—to allow for easy, tension-free recreation of the inframammary fold.] Flaps were debrided until they demonstrated brisk, bright red bleeding. We then brought the medial and lateral extents of each flap together into the breast meridian with suture, decreasing flap width but increasing projection [Video 1 (online)]. The RA was undermined off the rectus fascia to the umbilicus, sparing periumbilical perforators [Video 2 (online)], and quilted to minimize seroma and tension on the new IMF. Two drains were placed on each side. After the IMF was recreated, the RA tissue was placed into the prepectoral plane and stabilized with an adhesive bra for 6 days until follow-up (Fig. 3).
Video 1.
Second-stage reverse abdominoplasty surgery performed after first-stage Goldilocks mastectomy. This video demonstrates creation of the left breast “autologous implant” from excess tissue below the inframammary fold.
Video 2.
This is a video of a reverse abdominoplasty used to augment the left breast after Goldilocks mastectomy and flap reconstruction. Here we demonstrate how far we must undermine-down to the umbilicus but making sure to spare the periumbilical perforators-to allow for easy, tension free recreation of the inframammary fold.
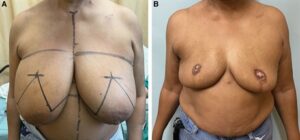 Fig. 1.:
Fig. 1.:
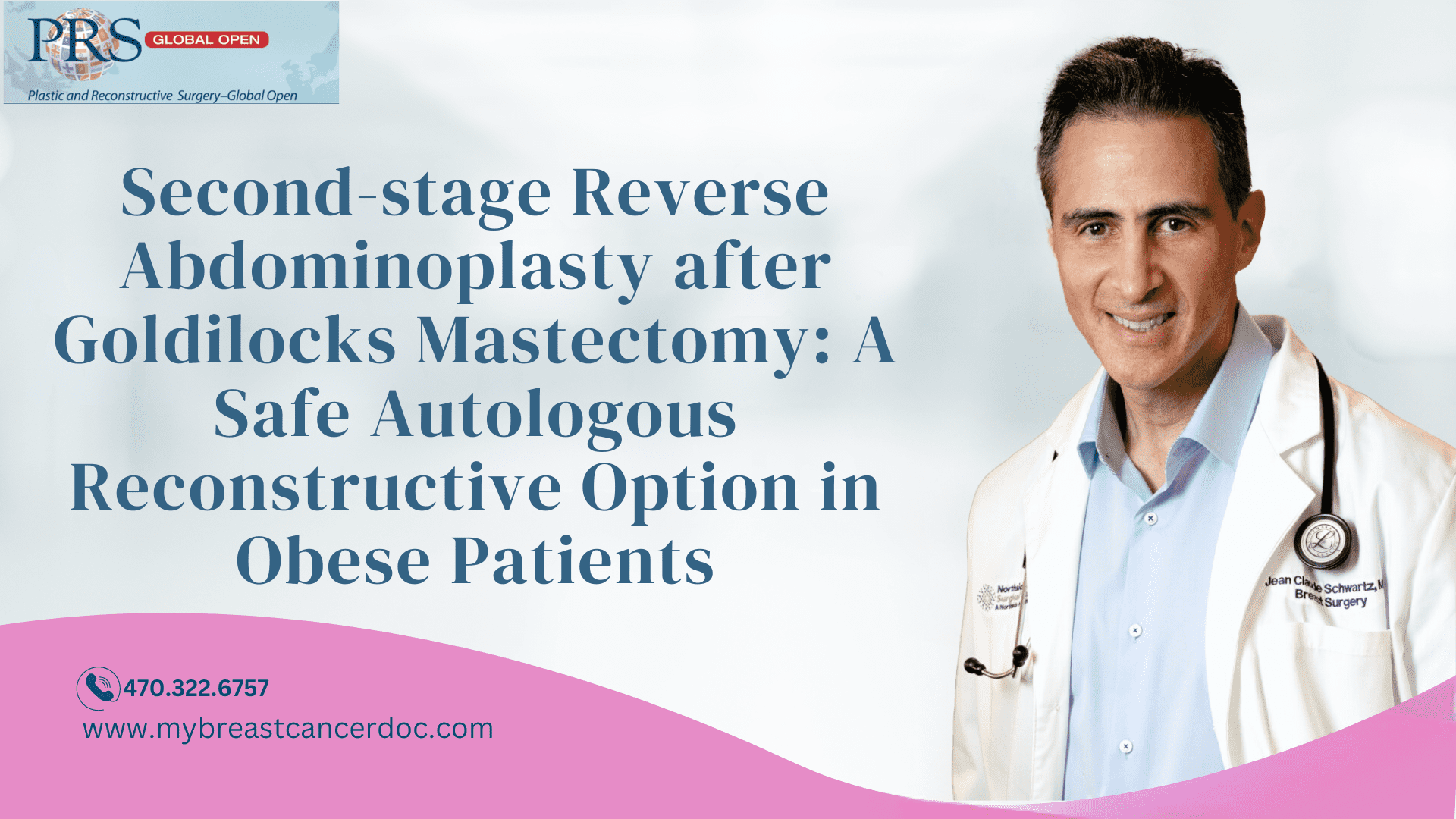
Pre- and postoperative photographs. A, A 52-year-old obese woman with a BMI of 34 kg per m2 presents with right breast cancer. She requests autologous reconstruction but refuses a deep inferior epigastric perforator flap after meeting with a microvascular surgeon. B, We proceed with a GM and free nipple grafts. She heals without complication but loses significant areolar pigmentation.
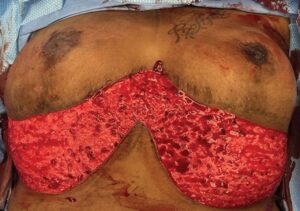 Fig. 2.:
Fig. 2.:
This is an intraoperative photograph from a second-stage RA. This is the deepithelialized adipodermal flap that will be divided in two and then placed into each breast for augmentation. Our approach is demonstrated in Video 1 (online).
 Fig. 3.:
Fig. 3.:
Postoperative photograph 6 months after RA. This patient healed without complication with larger, more aesthetic breasts. The only additional scar she requires after her first-stage GM is seen in the midline which bridges the two RA flaps and addresses the excess tissue in the midline. Although this additional scar is relatively small, it does tend to be hypertrophic in many women.
RESULTS
Fifteen patients underwent 10 bilateral and five unilateral GMs followed by RA. The mean patient age, BMI, and follow-up was 52 years (range, 32–66 years), 34.7 kg/m2 (range, 30.5–46 kg/m2), and 13 months (range, 6–30 months), respectively. Six (40%) and five (33%) patients had a diagnosis of hypertension and diabetes mellitus, respectively. The mean height, width, and thickness of the RA flaps were 9.5 cm (range, 6–12 cm), 20.0 cm (range, 17–25 cm), and 3.8 cm (range, 1.8–5.1 cm), respectively. Operative times for unilateral GM and contralateral reduction, bilateral GM, and second-stage RA were 182 minutes (range 157–260 minutes), 187 minutes (range, 168–277 minutes) and 110 minutes (range, 85–135 minutes), respectively. There were no postoperative abscesses or hematomas. Six (24%) and two (8%) first-stage GMs had wound dehiscence and mastectomy flap necrosis, respectively, which were treated with outpatient wound care. There were two (8%) and one (4%) first-stage GMs with seromas and cellulitis, respectively, requiring surgery and inpatient admission, respectively. Second-stage RA patients had two (13%) seromas and two (13 %) wound dehiscences, managed as outpatients. There was one (6%) patient with clinically apparent fat necrosis after RA that did not require intervention.
DISCUSSION
Autologous reconstruction in obese patients results in fewer complications and improved patient satisfaction.6 Despite these benefits, ~80% of reconstructions performed are implant-based.5 Implant-based methods are preferred for their simplicity, absence of donor site scarring and morbidity, abbreviated operative times, quicker recovery, and lack of available microvascular expertise.
Here, we present a two-stage autologous approach in ptotic, obese patients: first-stage GM followed by second-stage RA. We modify the approach by Zienowicz,8 who initially described cosmetic breast augmentation using the RA in normal BMI patients. Our patients require two outpatient procedures, providing them an autologous option without many of the disadvantages of and skillset required for microvascular reconstructions. The RA often improves upper abdominal contour with minimal additional scarring after Wise-pattern GM in contrast to the scarring after abdominal microvascular reconstructions, which may result in decreased patient satisfaction.9 [See figure, Supplemental Digital Content 1, which displays a 44-year-old obese (BMI = 43.2 kg/m2) woman status post right GM and left breast reduction 3 years ago at an outside institution. She reportedly had gigantomastia before surgery, although these photographs are unavailable. She requested an increase in breast size without the use of implants. Given her significant excess upper abdominal subcutaneous tissue, she is an ideal candidate for a reverse abdominoplasty. She is shown 3 months after her reverse abdominoplasty with improved upper abdominal contour and a significant increase in breast size. Postoperatively, she does have a right breast wound dehiscence which heals with outpatient wound care but does result in some depigmentation of her medial inframammary scar. She has some fullness centrally, which could be further debulked to improve her outcome. https://links.lww.com/PRSGO/D95.] Although we did not use fat grafting to the mastectomy flaps or RA, this combination could provide larger volumes to those marginal candidates for second-stage RA.
Although the exact volumes of the RA flaps were not calculated here, we feel their dimensions (9.5 cm X 20 cm X 3.8 cm) are consistent with volumes that approach the largest available prosthetics. While this report discussed the use of the RA after GM, we have used this technique to improve asymmetries after both traditional postmastectomy flap reconstructions and partial mastectomies (unpublished data) in obese patients. Although the RA has been presented in the literature predominantly as an aesthetic procedure7,8 or for closure after removing advanced cancers,10 it may be useful in obese patients, who have excess subcutaneous tissue in this region and are at high risk for complications after more standard methods of reconstruction.
Patients with concerns regarding their abdominal wall aesthetics are encouraged to proceed with abdominal microvascular reconstruction, as this addresses both excess upper and lower abdominal tissues. Patients who are focused strictly on improving their breast aesthetics are presented with both RA and abdominal reconstruction options.
We have not witnessed fat necrosis after division of the inferior Goldilocks “pedicle” and separation of this tissue from the pectoralis, leaving behind only skin attachments, to accommodate the RA. This is consistent with our experience with second-stage implant placement after first-stage GM.4
CONCLUSION
The GM followed by second-stage RA can provide obese patients a safe, autologous reconstruction without significant donor site scarring and morbidity, prolonged operative times or requirement for microvascular expertise.
DISCLOSURE
The author has no financial interest to declare in relation to the content of this article.
REFERENCES
Jean-Claude Schwartz, MD, PhD – Breast Cancer and Reconstructive Surgeon

As a national leader in oncoplastic breast surgery, Dr. Schwartz has developed and published some of the most innovative ways to save women’s breasts, allowing them to avoid mastectomy and multiple reconstructive surgeries. Dr. Schwartz’s techniques have benefitted his patients and other surgeons around the world.
There is no breast surgical oncologist in the United States that offers the same variety of oncoplastic options, nor has contributed more to the different fields of breast reconstruction than Dr. Schwartz.