Fax: 770-339-9804
Lawrenceville, Georgia 30046

Originally published on PRS Global Open website in December 2015
Abstract
Prepectoral implant-based breast reconstruction has become more common given the reduced dissection, decreased postoperative pain, elimination of animation deformity, and improved aesthetics compared with subpectoral reconstructions. Despite these benefits, surgeons must contend with increased rates of implant rippling and more tenuous implant positioning and coverage, especially in direct-to-implant reconstructions. Although the use of an acellular dermal matrix can assist with both implant rippling and support/positioning, it does not protect against implant exposure, and rippling can still occur, despite its use, with significant additional cost. This article looks into the use of a lateral adipodermal flap that assists with reducing the mismatch between the excess skin and implant surface area, implant positioning (by helping secure the lateral mammary fold), and providing implant coverage. Twenty-two patients underwent 38 nipple-sparing mastectomies and prepectoral direct-to-implant reconstructions using a lateral adipodermal flap without acellular dermal matrix. No patients had evidence of implant malposition or exposure with at least 6 months follow-up. The author concludes that the lateral adipodermal flap may be helpful in securing the lateral mammary fold, reducing excess skin and providing viable tissue coverage in patients undergoing prepectoral direct-to-implant reconstruction.
Takeaways
Question: How can we make use of lateral breast skin excess when performing prepectoral direct-to-implant reconstruction?
Findings: This study describes 39 nipple-sparing mastectomies undergoing prepectoral direct-to-implant reconstruction using a laterally-based adipodermal flap to assist with implant coverage and positioning. All patients successfully underwent breast reconstruction without the use of acellular dermal matrix, with no instances of lateral implant malposition. Only two patients requested revision for implant rippling.
Meaning: A laterally-based adipodermal flap can improve outcomes in patients undergoing prepectoral direct-to-implant reconstruction by removing excess skin and assisting with both implant positioning and coverage.
INTRODUCTION
Prepectoral reconstructions minimize postoperative pain, eliminate animation deformity, and have reduced recovery time.1 Despite these benefits, there is less implant coverage, and larger amounts of acellular dermal matrix (ADM) are required for positioning/support. This support is critical in direct-to-implant reconstruction (DTIR) where full-sized implants are stabilized within the breast footprint, which is often compromised by the mastectomy.2 Optimal results are facilitated by a hand-in-glove fit between the skin envelope and implant, which can be difficult in larger breasts. In ptotic breasts, excess skin is best addressed using a Wise pattern, but this is often not possible after previous breast reduction/mastopexy or in larger nonptotic breasts.3
We have appreciated that most female breasts have lateral breast skin excess (LBSE) (Figs. 1 and 2). (See Video 1 [online], which displays the preoperative planning, creation of the lateral adipodermal flap and mastectomy.) This excess skin can be deepithelialized to create a lateral adipodermal flap (LAF) that can be sutured down to the chest wall to precisely accommodate the implant width (Fig. 3). (See Video 2 [online], which displays the reconstruction and securing of the lateral adipodermal flap to the lateral mammary fold.) (See Video 3 [online], which displays the final on-table result demonstrating the reconstructed breast with implant secured using the lateral adipodermal flap.) This approach was developed where ADM was not available but can be used in all patients. In addition to securing the lateral mammary fold (LMF), this technique tightens up the skin envelope and provides tissue coverage when the flap is medially-based. Here, the author presents a series of patients undergoing nipple-sparing mastectomy (NSM) and prepectoral DTIR where this approach is utilized.
Video 1.
This video displays the preoperative planning and creation of the lateral adipodermal flap and mastectomy.
Video 2.
This video displays the reconstruction and securing of the lateral adipodermal flap to the lateral mammary fold.
Video 3.
This video displays the final on-table result demonstrating the reconstructed breast with implant secured using the lateral adipodermal flap.
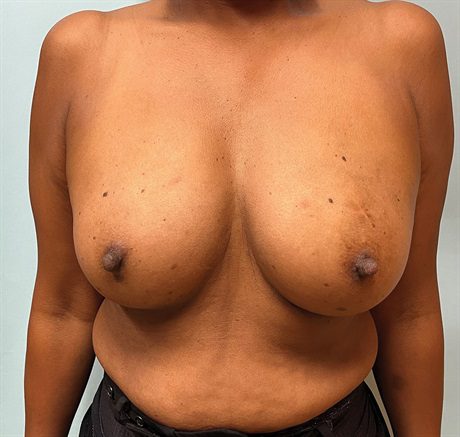 Fig. 1.:
Fig. 1.:
A 53-year-old woman with multicentric left breast cancer requiring mastectomy. She has a history of multiple benign left breast biopsies. She has no significant ptosis and has a larger breast volume.
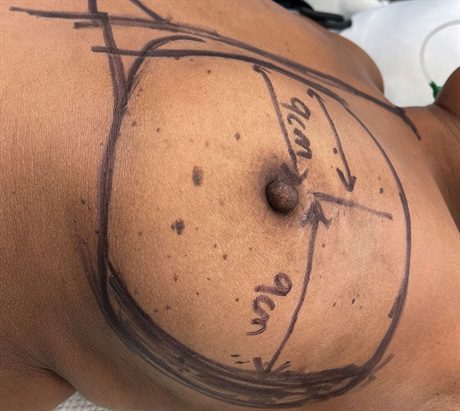 Fig. 2.:
Fig. 2.:
Preoperative markings demonstrating a 9-cm distance from the lateral sternal border to the nipple. The distance from the nipple to the anterior axillary line is 12 cm, leaving us with 3 cm of LBSE that can be deepithelialized. The lateral crescent is filled in with black marker.
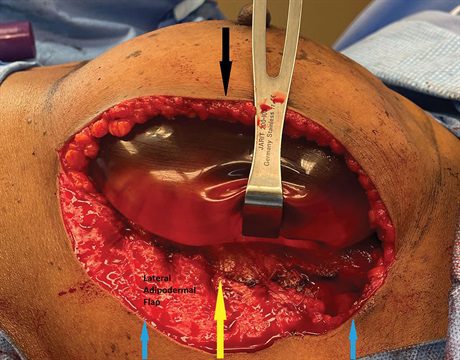 Fig. 3.:
Fig. 3.:
Here we see an intraoperative photograph of the LBSE or lateral crescent that has been deepithelialized to form the lateral adipodermal flap after mastectomy. Her mastectomy specimen weighs 680 g, necessitating a large implant. The lateral adipodermal flap has been secured to the new lateral mammary fold (yellow arrow). This position is determined by the base width of the chosen implant. No acellular dermal matrix is used. The new lateral mastectomy flap skin edge (black arrow) is then sutured down to the new lateral mammary fold. The wound is then closed. By leaving the same amount of skin from the lateral sternal border to the nipple and from the nipple to the new lateral skin edge to cover the precise base width of the implant, the nipple is not laterally displaced. Lateral displacement would occur if the new lateral skin edge was just sutured to the lateral base of the crescent (blue arrows) instead of medializing over toward where the lateral edge of the implant will lie (yellow arrow).
METHODS
Surgical Technique
Retrospective review of all NSM and prepectoral DTIRs using the LAF with at least 6 months follow-up was performed. Patients who had LBSE with grades 0,1 and mild grade 2 ptosis were included. In those patients with LBSE who did not require a Wise pattern, a medial (see Videos 1–3) or lateral (Figs. 2 and 3) crescent of skin was deepithelialized, through which the mastectomy was performed and LMF reconstructed. Medially-based LAFs better assist with implant coverage. No ADM was utilized in this series but can be combined with the LAF (see Videos 1-3). The lateral thoracic tissue is advanced and secured to the LMF for further reinforcement and to close off dead space. All implants used were Mentor (Santa Barbara, Calif.). Memory Gel XTRA smooth round moderate plus or moderate high profile. The final scar is not visible in the anterior-posterior view (Fig. 4) but can be seen from the side. (See figure, Supplemental Digital Content 1, which shows a 13-month postoperative photograph, 45-degree view of the patient. https://links.lww.com/PRSGO/C451.)
RESULTS
Twenty-two patients underwent 16 bilateral and six unilateral NSMs with prepectoral DTIRs using the LAF. The mean patient age, body mass index, and follow-up was 54 years (range, 24–71 years), 28.7 kg/m2 (range, 19–42 kg/m2) and 11 months (range, 6–30 months), respectively. Thirteen (59%) patients had undergone previous breast reduction/mastopexy for symptomatic macromastia or cancer and had an average LBSE of 4.7 cm (range, 3–8 cm), average sternal notch to nipple distance of 24.5 cm (range, 19–28 cm), average nipple to inframammary fold distance of 9.2 cm (range, 8–12 cm), and were reconstructed with implants which had an average volume of 705 cm3 (range, 605–800 cm3). The remaining nine patients, with no previous history of surgery, had an LBSE of 3.1 cm (range, 2–5 cm), average sternal notch to nipple distance of 22.4 cm (range, 18–24 cm), average nipple to inframammary fold distance of 7.4 cm, and were reconstructed with implants that had an average volume of 605 cm3 (range, 520–790 cm3). Twenty-seven LAFs were medially-based, and 11 were laterally-based. Three (7.9%) breasts developed periprosthetic infections and underwent salvage with implant replacement. There were three (7.9%) seromas, with two (5.2%) requiring operative drainage. Three (7.9%) breasts developed wound dehiscence, of which one (2.6%) underwent immediate surgical revision but the remainder healed with wound care. Two (5.2%) breasts developed mastectomy flap necrosis, both of which underwent successful surgical revision. Three breasts (7.9%) have undergone revision for implant rippling. Four (10.4%) breasts underwent postmastectomy radiotherapy with no obvious deleterious impact on the final aesthetic outcome.
DISCUSSION
The Wise pattern is most applicable for patients with ptosis and skin excess who undergo mastectomy and reconstruction.4 However, not all patients with skin excess benefit from a Wise-pattern skin reduction. Larger breasts without significant ptosis do not require a Wise-pattern but still have sizable skin envelopes with significant LBSE that benefit from reduction (unpublished results). After previous reduction/mastopexy, many patients do not have significant ptosis but develop significant LBSE (unpublished results). These patients still have large skin envelopes that are often incompletely filled, even with the largest implants.
Most patients in the age group that develop breast cancer, regardless of breast size, have some element of LBSE. This LBSE can be deepithelialized, used as access to perform the mastectomy, and can help define the LMF. In addition, this lateral skin tightening allows for a better fit between the skin envelope and implant. Medially-based LAFs also assist with implant coverage to prevent exposure (see Videos 1-3).
The durability of implant reconstructions is often debated with lower pole support recommended to prevent bottoming out.5 The author believes that while bottoming out is certainly an important concern, compromise of the LMF is also often overlooked, leading to implant malposition and poor long-term results. The LAF allows us to secure and reinforce the LMF, much like a Ryan flap6 does for the inframammary fold, potentially obviating the immediate need for ADM and simultaneously reducing the skin envelope to better accommodate the implant and also providing tissue coverage to reduce the risk of exposure.
Although this approach was developed to provide LMF support in patients where ADM was not available, the author does not make the claim that it has comparable long-term outcomes with respect to capsular contracture, rippling, and lower pole and LMF support. Previous publications better address the issue of whether ADM is necessary in prepectoral reconstructions.7–10 This approach may be of special relevance in less-developed countries where ADM cost may be prohibitive. The author presents this as a new, simple approach that may be of benefit in some patients with LBSE and is likely complementary to other strategies (ADM, fat grafting, cohesive implants, preserving of the oncoplastic plane) to improve aesthetic outcomes. The long-term durability of this approach requires larger patient numbers with longer follow-up.
 Fig. 4.:
Fig. 4.:
Thirteen months follow-up photograph after the patient’s direct-to-implant reconstruction. There is excellent symmetry between the breasts with no evidence or rippling or implant malposition. Her mastectomy scar is not visible in the anterior-posterior view but can be appreciated from the side (Supplement Digital Content 1, https://links.lww.com/PRSGO/C451).
REFERENCES
Jean-Claude Schwartz, MD, PhD – Breast Cancer and Reconstructive Surgeon

As a national leader in oncoplastic breast surgery, Dr. Schwartz has developed and published some of the most innovative ways to save women’s breasts, allowing them to avoid mastectomy and multiple reconstructive surgeries. Dr. Schwartz’s techniques have benefitted his patients and other surgeons around the world.
There is no breast surgical oncologist in the United States that offers the same variety of oncoplastic options, nor has contributed more to the different fields of breast reconstruction than Dr. Schwartz.