Fax: 770-339-9804
Lawrenceville, Georgia 30046

Originally published on PRS Global Open website in March 2024
Abstract
Summary:
Smaller breasts require volume replacement to allow for breast conservation for large tumor to breast size ratios. The lateral intercostal artery perforator (LICAP) flap is one of the most commonly used approaches to replace volume as it readily fills lateral breast defects where most cancers typically arise. The LICAP flap was initially described with the bulk of its tissue volume oriented posteriorly, lateral to the breast footprint. Recently, the “reverse LICAP” flap was described, which uses the same perforators but recruits tissue instead from below the inframammary fold. Here, we combine these two approaches, preserving the same perforators, but harvesting tissue from both below the inframammary fold and lateral to the breast footprint, to create a single, larger, bidirectional LICAP flap. This modified flap replaces more volume than previously described for uni-directional approaches allowing us to potentially avoid mastectomy or more extensive flap reconstructions. Here, we describe 10 consecutive patients where the bidirectional LICAP flap was used to facilitate oncoplastic breast conservation.
Takeaways
Question: How can we modify the lateral intercostal artery perforator (LICAP) flap to recruit additional tissue to aid in reconstructing larger partial mastectomy defects?
Findings: This study demonstrated that by combining the traditional LICAP flap with a recently described “reverse LICAP” into one larger bidirectional flap, we can harvest additional tissues to aid in replacing volume after partial mastectomy.
Meaning: The bidirectional LICAP flap provides surgeons additional volume than the traditional LICAP flap, allowing surgeons to avoid mastectomy or larger, more morbid flap procedures that would be required for breast conservation.
INTRODUCTION
Oncoplastic breast conservation is divided into volume replacement (VR) and volume displacement approaches.1 Local chest wall perforator flaps [such as the lateral intercostal artery perforator (LICAP), medial intercostal artery perforator (MICAP) and anterior intercostal artery perforator (AICAP) flaps] minimize morbidity by allowing us to use smaller, local flaps that do not require intraoperative position changes or exhaust a major reconstructive modality.2,3 Although these flaps are less morbid than a latissimus dorsi (LD) flap or a thoracodorsal artery perforator flap, their major drawback is smaller available volumes. As we extend the indications for breast conservation and embrace “extreme oncoplasty,”4 surgeons will require larger amounts of tissue to reconstruct more extensive breast defects. Here, we describe the bidirectional LICAP (bLICAP) flap, which incorporates tissue from below the inframammary fold (IMF) and lateral to the breast footprint into a single larger flap, providing a simpler approach to VR than flaps harvested from the back or other distant sites.
PATIENTS AND METHODS
We retrospectively reviewed the charts of patients who underwent partial mastectomy (PM) and immediate reconstruction with the bLICAP flap from January 2021 through March 2023 with at least 6 months follow-up. All procedures were performed by the author.
The following patient variables, operative details, and complications were recorded: age; body mass index (BMI); breast ptosis grade; breast cup size; operative duration; PM specimen weight and tumor size; height, width, and thickness of the bLICAP flap; major and minor complications; rate of clinical flap fat necrosis (defined a palpable hardening in the reconstructed breast with at least 6 months follow-up); and length of follow-up.
OPERATIVE TECHNIQUE
Patients were candidates for this approach if they had a tumor in the lateral breast that was deemed too large for level I oncoplastic techniques, which involve simple adjacent tissue transfer of intramammary flaps, and did not have significant ptosis for level II techniques, which rely on mammoplasty techniques.1 Briefly, the patients are marked in the standing position (Fig. 1), and perforator locations are determined with an 8-megahertz Doppler in the supine position. After the PM is performed (Fig. 2), the need for a standard LICAP, reverse LICAP, or bLICAP is typically assessed after confirmation that a simple adjacent tissue transfer cannot reconstruct the defect. With the weight of the specimen giving us an approximate volume, we calculate the volume available for each of these three flaps (by multiplying height x width x thickness) and then decide on which flap is appropriate. After confirmation of the need for a bLICAP, excess subcutaneous tissue below the IMF and a transversely oriented extension to the anterior border of the LD were included in the flap (Fig. 3). The flap was dissected of the underlying muscle both from its most medial point near the sternum and lateral point near the anterior border of the LD, converging on the LICAP in the central part of the flap [see Video 1 (online)]. We then reconstructed the IMF using a standard reverse abdominoplasty approach and closed the remainder of the donor site lateral to the breast footprint before insetting the flap (Fig. 4). A drain was placed into the breast which was removed at the first follow-up visit. [See Video (online), which shows the dissected bidirectional LICAP flap with identification of perforators and reconstruction of the partial mastectomy defect.]
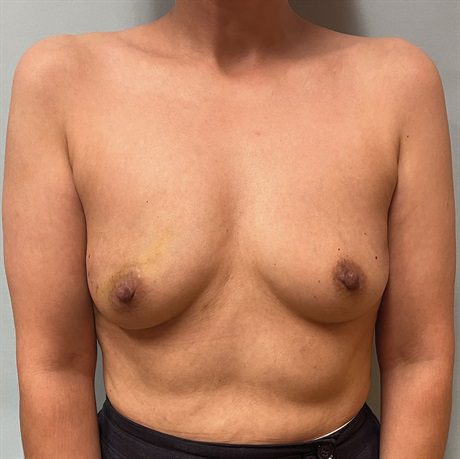 Fig. 1.:
Fig. 1.:
A 50-year-old woman (body mass index 21.5 kg/m2), with 5 cm left breast DCIS who desires breast conservation. Given her small breast size and minimal ptosis, she is not a candidate for level I or level II approaches, respectively. We feel that she is a marginal candidate for a standard local chest wall perforator flap given her minimal excess subcutaneous tissue both inferior and lateral to her breast footprint and would require a thoracodorsal artery perforator flap, which she refuses. We offer her bidirectional LICAP flap to immediately reconstruct her breast.
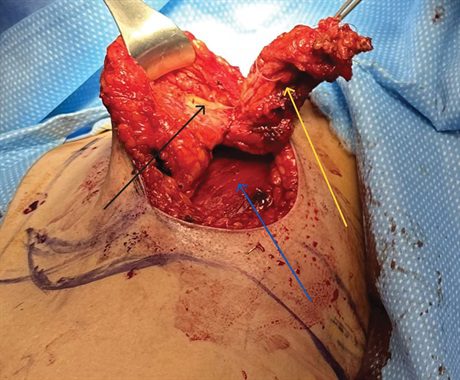 Fig. 2.:
Fig. 2.:
This photograph demonstrates the extensive PM (yellow arrow), which is performed from the upper outer quadrant of her breast. The resection extends from the chest wall (blue arrow) to dermis (black arrow). The patient’s breast is then reconstructed using the bidirectional LICAP flap (see Video).
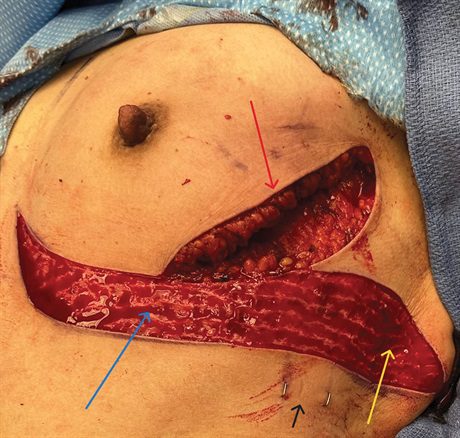 Fig. 3.:
Fig. 3.:
The de-epithelialized bidirectional LICAP flap is depicted here in this intraoperative photograph. Tissues from both below the inframammary fold (blue arrow) and lateral to the breast footprint (yellow arrow) are included in the flap. The staples mark the two perforators (black arrow) which were identified preoperatively with Doppler. The additional incision along the lateral mammary fold (red arrow) provides additional access for her partial mastectomy and lymph node dissection.
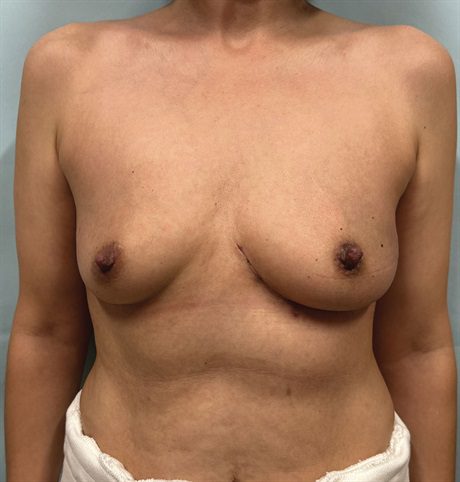 Fig. 4.:
Fig. 4.:
This photograph demonstrates the patient’s postoperative result 6 months after the completion of whole breast radiotherapy. Although her reconstructed breast is slightly larger than the contralateral side, it demonstrates no evidence of deformity. This approach allows her to avoid a mastectomy or a more complex flap reconstruction to save her breast.
Video 1.
This video shows the dissected bi-directional LICAP flap with identification of perforators and reconstruction of the partial mastectomy defect.
RESULTS
Ten patients underwent PM and immediate bidirectional LICAP flap reconstruction. The mean patient age, BMI, and follow-up was 54 years (range, 38–64 years), 23.7 kg/m2 (range, 20–32 kg/m2), and 9 months (range, 6–20 months), respectively. Mean PM weight was 125 g (range, 55–230 g). The mean height, width and thickness of the bLICAP flaps were 3.1 cm (range, 2–5 cm), 19.8 cm (range, 15–25 cm) and 1.7 cm (range, 1–4 cm), respectively. Each patient’s preoperative breast cup size, breast ptosis grade, specimen weight, tumor size, and flap dimensions are presented. (See table, Supplemental Digital Content 1, which shows demographics, breast cup size, breast ptosis grade, specimen weight, tumor size and flap dimensions for each patient undergoing lumpectomy and bidirectional LICAP flap reconstruction, https://links.lww.com/PRSGO/D114.) There were no postoperative abscesses, hematomas or incidence of cellulitis. There was one (10%) donor site dehiscence that healed with outpatient wound care and one (10%) seroma that required operative drainage. The average follow-up time was 9 months. There was one (10%) patient with clinically apparent fat necrosis that did not require intervention.
DISCUSSION
Oncoplastic techniques allow surgeons to immediately reconstruct breast defects, reducing the chance of breast deformity.1 Smaller, nonptotic breasts rely on VR,2,3,5 which recruits nonbreast tissue to reconstruct PM defects. The optimal VR techniques require short operative times, do not exhaust a major reconstructive modality, do not have significant donor site complications, are within the scope of practice of most reconstructive surgeons, and provide enough tissue to reconstruct larger defects. Although the AICAP, MICAP, and LICAP flaps satisfy most of these criteria, the volume they provide is typically limited, especially in thinner women. Here, we describe an approach to combine the tissue that is recruited when an AICAP or MICAP flap is raised (submammary tissue) with that from a traditional LICAP flap (lateral to the breast footprint), to create a larger flap that allows us to replace larger volumes with minimal additional operative complexity, time, or morbidity. The ability to reconstruct larger defects is becoming more relevant as surgeons document improved survival and decreased recurrence rates for breast conservation versus mastectomy6 and the safety of breast conservation for larger, multicentric tumors.7
Spinelli et al8 recently described the “reverse LICAP” flap where tissue from below the IMF based off LICAPs is used for postmastectomy reconstruction and other benign conditions. They did not address immediate reconstruction of PM defects. We have used reverse LICAPs (unpublished data) for reconstructing PM defects primarily for ease of access (no position change required which on occasion is necessary for LICAP flaps) and for defects where the standard LICAP turnover flap might leave additional lateral bulk.
We have not witnessed significant issues with vascularization of these flaps as others have described with “extended LICAPs” where far greater volumes of tissues are harvested from the back supplied by these same perforators.9,10 Like traditional LICAP flaps, bLICAP flaps are primarily useful for lateral defects and may require a minor position change to recruit tissues lateral to the breast footprint.
DISCLOSURE
The author has no financial interest to declare in relation to the content of this article.
REFERENCE
Jean-Claude Schwartz, MD, PhD – Breast Cancer and Reconstructive Surgeon

As a national leader in oncoplastic breast surgery, Dr. Schwartz has developed and published some of the most innovative ways to save women’s breasts, allowing them to avoid mastectomy and multiple reconstructive surgeries. Dr. Schwartz’s techniques have benefitted his patients and other surgeons around the world.
There is no breast surgical oncologist in the United States that offers the same variety of oncoplastic options, nor has contributed more to the different fields of breast reconstruction than Dr. Schwartz.