Fax: 770-339-9804
Lawrenceville, Georgia 30046


Originally published on PRS Global Open website in December 2015
Summary: Outstanding results are difficult to achieve in postmastectomy reconstructions in obese ptotic patients. We describe an autologous single-stage reconstruction with free nipple grafts that is best suited for these difficult patients. This technique allows for delayed volume supplementation with implants or fat grafting but does not commit the patient to additional surgery. It avoids the common complications of immediate implant-based reconstructions. This technique is also an excellent option in patients with a known requirement for radiotherapy as it does not sacrifice a valuable autologous flap nor does it subject the patient to capsular con-tracture, infection, and extrusion. It also obviates the psychological trauma that many women suffer awaiting a reconstruction after radiotherapy. We believe it should be considered as a first-line reconstructive option.
Jean-Claude D. Schwartz, MD, PhD*, Piotr P. Skowronski, MD†
A 67-year-old woman had 10 cm of grade 3 ductal carcinoma in situ in her right breast. She had significant macromastia and ptosis (Fig. 1). She decided to proceed with bilateral mastectomy and wanted some form of reconstruction. She is a diabetic (Hg- bA1c 7.1) with a body mass index of 37 making her a high-risk candidate for any immediate implant or flap-based reconstruction. Given her obesity, macromastia, and ptosis, we felt that we could use the Goldilocks mastectomy to provide her with adequate breast volume to reconstruct her final breast mound in one stage. In addition, we planned on salvaging her left NAC as a free nipple graft and using the excess areolar skin to reconstruct the right NAC (proximity to cancer precluded saving this).
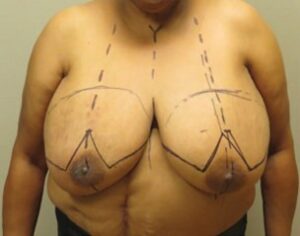
Fig. 1. A 67-year-old woman with multicentric right breast ductal carcinoma in situ. She is obese with significant macromastia and ptosis. She is marked with a standard Wise pattern before surgery.
In the preoperative holding area, the patient was marked with standard bilateral Wise reduction patterns (Fig. 1). The right mastectomy was performed by incising the medial and lateral extensions to the inframammary fold and excising the NAC. Flaps were developed in standard fashion with care taken to completely remove the breast tissue but to preserve the subcutaneous layer. All of the skin within the mammoplasty markings was carefully de-epithelialized.
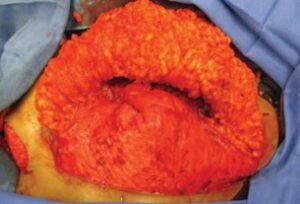
Fig. 2. Reconstruction of the breast mound using the inferior mastectomy flap dermis and fat. It is folded on itself transversely and then vertically and centered over the meridian. Dermis is secured to dermis using 2-0 PDS and then sutured down to the pectoralis muscle.
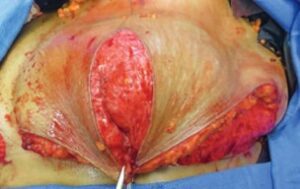
Fig. 3. The superior mastectomy flap is brought down over the reconstructed breast mound to give the breast additional volume and projection. This closure is virtually identical to the standard closure of an inferior pedicle breast reduction.
The inferior breast flap was separated from the superior flap. This allowed us to mold the inferior flap to recreate a breast mound. It was folded on itself transversely and then folded again vertically. A 2-0 polydioxanone suture (PDS) was used to create the mound securing dermis to dermis. This tissue was then shifted medially, so the bulk of the mound was centered over the meridian. This was also then secured to the pectoralis major muscle using an additional PDS suture (Fig. 2). The superior mastectomy flap was then draped over the inferior breast mound much as we close an inferior pedicle breast reduction (Fig. 3). The vertical limbs are then brought together giving the reconstructed breast additional projection from the bulk supplied by the dermis and fat located between the limbs. The left prophylactic mastectomy and reconstruction were performed in exactly the same fashion. The left NAC is used as a free nipple graft to reconstruct the left breast, and the excess left areolar skin is used to reconstruct the right NAC. The final on-table result is a completed single-stage autologous reconstruction (Fig. 4). There is no doubt that in many patients with sufficient macromastia, ptosis, and a reasonable amount of adipose tissue, there exists sufficient volume to reconstruct breast mounds in a single-stage.
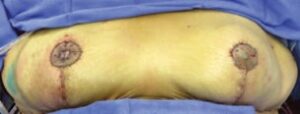
Fig. 4. The final on-table result after free nipple grafting the left breast and reconstructing the right NAC with excess left areolar skin and a disk of the right breast skin.
These breast mounds are aesthetic in appearance as the skin envelope has been retailored using a Wise pattern, and the excess dermis and fat are sculpted into a conical shape and centered over the meridian competing very favorably with traditional reconstructions. We also feel that NAC reconstruction with free nipple grafts should be a standard practice un- less the patient refuses or oncological considerations preclude this. This case report describes an immediate, definitive postmastectomy reconstruction in a single operation that routinely takes multiple surgeries to complete using other techniques. Many of these patients are not interested in such a long reconstructive process or are at high risk for multiple general anesthetics.
There are many patients who demand a completely autologous reconstruction and are not good candidates for bilateral abdominal-based autologous flaps. The surgery described here with the option of delayed fat grafting can provide this patient population with a completely autologous option with significantly less operative time, fewer complications, no donor site scars, and without the need for microvascular surgery expertise.
This strategy described here coupled with a second-stage definitive implant may also become the preferred standard practice in many patients with macromastia, ptosis, and obesity when compared with the traditional expander-based reconstructions followed by definitive implants. This approach avoids the complications of immediate implant-based reconstructions, namely expander infection, and extrusion, and probably minimizes the chances of capsular contracture as the definitive implant can be placed in a sterile, blood-free environment avoiding the immediate postmastectomy milieu. This patient population is at the highest risk for infection and implant extrusion, which can be minimized with this strategy.8
Finally, this strategy may play an important role in this population of patients who require postmastectomy radiotherapy. Postmastectomy reconstruction in the face of a known requirement for radiotherapy is a challenging and controversial area often with poor and unpredictable results, frequently requiring flap surgery.9–11 We feel that patients who undergo our modified Goldilocks surgery may tolerate radiotherapy in a manner similar to that of breast conservation patients, with minimal impact on the shape
and suppleness of the reconstruction. The option of delayed fat grafting can provide additional volume and enhance mastectomy flap health after radio-therapy is completed. We feel that this is a promising technique that requires careful study and follow-up to determine long-term results in comparison with our current standard practices.
REFERENCES
Jean-Claude Schwartz, MD, PhD – Breast Cancer and Reconstructive Surgeon

As a national leader in oncoplastic breast surgery, Dr. Schwartz has developed and published some of the most innovative ways to save women’s breasts, allowing them to avoid mastectomy and multiple reconstructive surgeries. Dr. Schwartz’s techniques have benefitted his patients and other surgeons around the world.
There is no breast surgical oncologist in the United States that offers the same variety of oncoplastic options, nor has contributed more to the different fields of breast reconstruction than Dr. Schwartz.